IntroductionCauses and SymptomsThe human BCS1L geneCase reportsEpidemiologyDiagnosis and TreatmentReferences A rare condition, Bjornstad syndrome is associated with mutations in the BCS1L gene. The two hallmark features of the syndrome include pili torti and sensorineural hearing loss. Professor Bjornstad first described it in 1965. The syndrome follows the autosomal recessive pattern of inheritance. Causes and Symptoms […]
Home » Health Problems »
Immunity boosting treatment enhances CAR-T cell therapy for blood cancers
Advances in cellular immunotherapy that spur genetically modified T cells to attack cancer cells have revolutionized the treatment of certain blood cancers. Six such CAR-T cell therapies are approved by the Food and Drug Administration to treat certain types of leukemia, lymphoma and multiple myeloma. Still, some patients’ tumors don’t respond well to these therapies, […]
Using Real-World Data to Accelerate Healthcare
IntroductionWhat is Real World Data?Experimental Data vs. RWDDifficulties with RWDBenefits of RWD Real-World Effectiveness Drug Repurposing Safety EvaluationWhat Lies Ahead for RWD?References Healthcare is more of an art than a science since people are so different from each other. Today's gold standard of safety, potency, and efficacy regarding healthcare practices is the […]
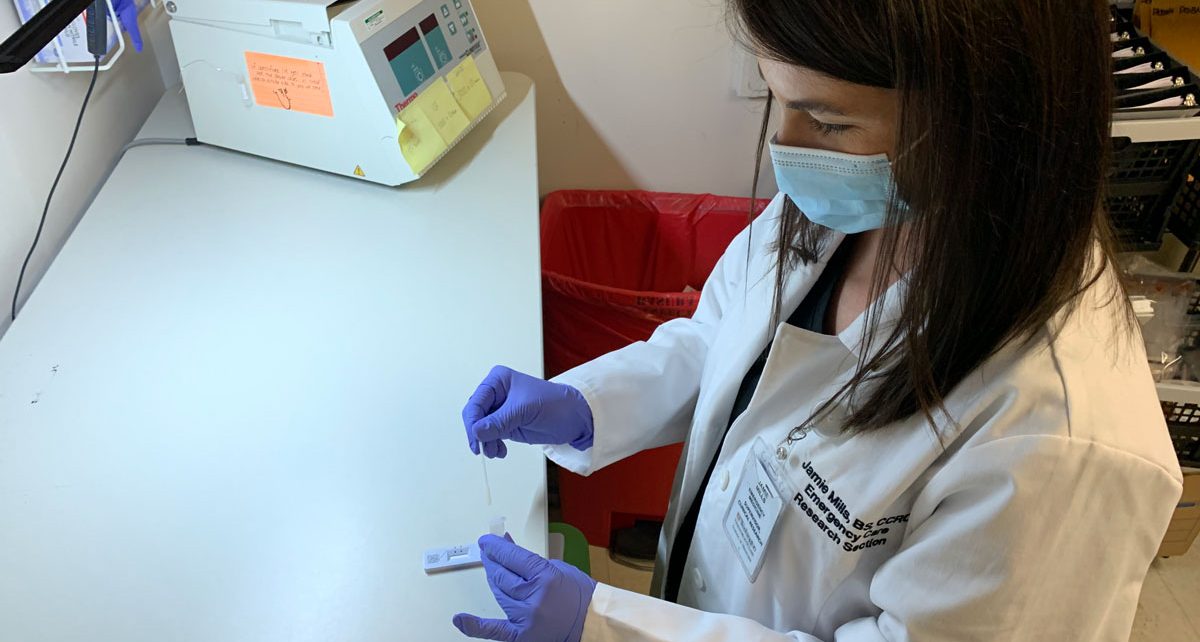
Emergency department plays key role in evaluating COVID-19 tests
More than two years into the COVID-19 pandemic, most Americans likely share the experience of anxiously awaiting the results of a COVID test, whether a lab-based PCR test administered at a hospital, a “point-of-care” rapid test given at an urgent care clinic, or an over-the-counter test taken at home. Starting in spring 2020, as emergency […]
Synthetic data mimics real patient data, accurately models COVID-19 pandemic
While caring for COVID-19 patients, health-care professionals across the country have amassed a treasure trove of information about SARS-CoV-2, its evolving variants such as delta and omicron, and their effects on the human body and public health. Such data, collected in patients’ electronic medical records, are vital for understanding the virus and developing treatments. But […]
Risky driving behaviors increase as common sleep disorder worsens
People with sleep apnea wake up tired in the morning, no matter how many hours they actually sleep. The condition causes them to briefly stop and restart breathing dozens or even hundreds of times a night. Even though such breathing interruptions often don’t awaken those with apnea, they prevent them from sinking into deep, refreshing […]
Recent Advancements in Treatment for Parkinson’s Disease
Parkinson’s disease (PD) is among the most common neurodegenerative disorders. Classified as a movement disorder, it is characterized by bradykinesia, rigidity, rest tremor, and postural instability. The treatment of this condition is currently centered on dopaminergic tone in the corpus striatum of the brain since a shortfall in dopamine stimulation of these neurons is thought […]
What is the Human Virome?
The Virome: A New Frontier in Physiological Understanding Since the beginning of the COVID-19 pandemic, terms such as “virology” and “immunity” have become constant mainstays on virtually all communication platforms. As such, the general population has become highly aware of the threat posed by highly contagious viruses and the measures we can take to hamper […]
Importance of Research into Rare Disease
According to Global Genes, around 7,000 rare diseases affect approximately 350 million people worldwide. Millions of these people are still waiting and hoping for effective treatment. As the name suggests, rare diseases only affect a small fraction of the global population, for instance, in Europe individuals with rare diseases occur in the ratio of 1 […]
The Effect of Diet on Mental Health
The brain controls and regulates most of the body’s vital functions, conscious or not. For this reason, it is essential that the brain receives a steady supply of fuel and oxygen. The fuel is obtained by metabolizing nutrients made available in the bloodstream, originating in the digested food. The brain consumes 20% of the daily […]