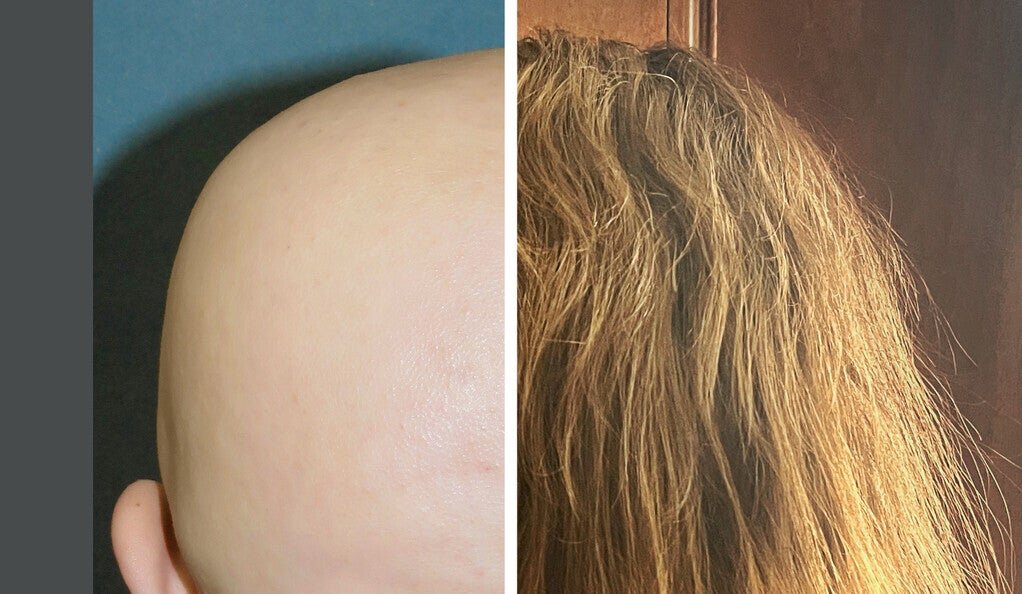
A medication that has been found to effectively treat the skin disease alopecia areata in adults is also successful in treating adolescent patients, according to a Yale-led clinical trial.
Alopecia areata is an autoimmune disease characterized by sudden, often disfiguring, loss of hair. It is the second most common cause of hair loss, affecting approximately 7 million people in the United States.
The new oral medication studied in the clinical trial, a Janus kinase (JAK) inhibitor known as ritlecitinib, was developed by Pfizer. Other JAK inhibitors, a class of drugs originally used to treat rheumatoid arthritis and certain blood disorders, have been approved for the treatment of a host of intractable skin diseases, including alopecia areata, following more than a decade of research led by Yale dermatologist Dr. Brett King.
Last year, the U.S. Food and Drug Administration approved the use of the JAK inhibitor baricitinib to treat severe alopecia areata—but only in adults. King, who was the lead investigator in that effort, also headed up the current study with ritlecitinib.
“This new work is a huge advancement for treating alopecia areata because the clinical trial involved adolescents in addition to adults,” said King, an associate professor of dermatology at Yale School of Medicine and first author of the new study, which was published in The Lancet. “Because alopecia areata frequently affects children and adolescents, it is groundbreaking to advance a medicine that shows safety and effectiveness in the treatment of younger patients.”
The phase 3 trial followed 718 patients—including more than 100 adolescent patients—at 118 hospitals and clinics in 18 countries. All the participants, regardless of their age, had at least 50% scalp hair loss due to alopecia areata.
After 24 weeks of ritlecitinib use, many patients experienced complete or near-complete regrowth of scalp hair, the study found. With continued ritlecitinib use for an additional 24 weeks, more patients achieved hair regrowth. The medication was well-tolerated in patients over the course of the study.
Significantly, King said, the results were consistent among all age groups, including younger patients.
“Alopecia areata often causes enormous suffering, for adults and kids alike,” King said. “Being a kid is hard enough as it is, so imagine what it’s like to be a kid with big bald spots, maybe missing an eyebrow, maybe without any hair at all. It can be punishing.”
King noted that a young girl with severe alopecia areata recently took her own life because she was being bullied at school. “We don’t want for that to ever happen again,” he said. “We need to do everything we can to ensure it doesn’t, and one part of that is advancing medicines to reverse disease. This study of ritlecitinib is a big step in that direction.”
A longer-term study of ritlecitinib to treat alopecia areata is ongoing.
More information:
Brett King et al, Efficacy and safety of ritlecitinib in adults and adolescents with alopecia areata: a randomised, double-blind, multicentre, phase 2b–3 trial, The Lancet (2023). DOI: 10.1016/S0140-6736(23)00222-2
Journal information:
The Lancet
Source: Read Full Article