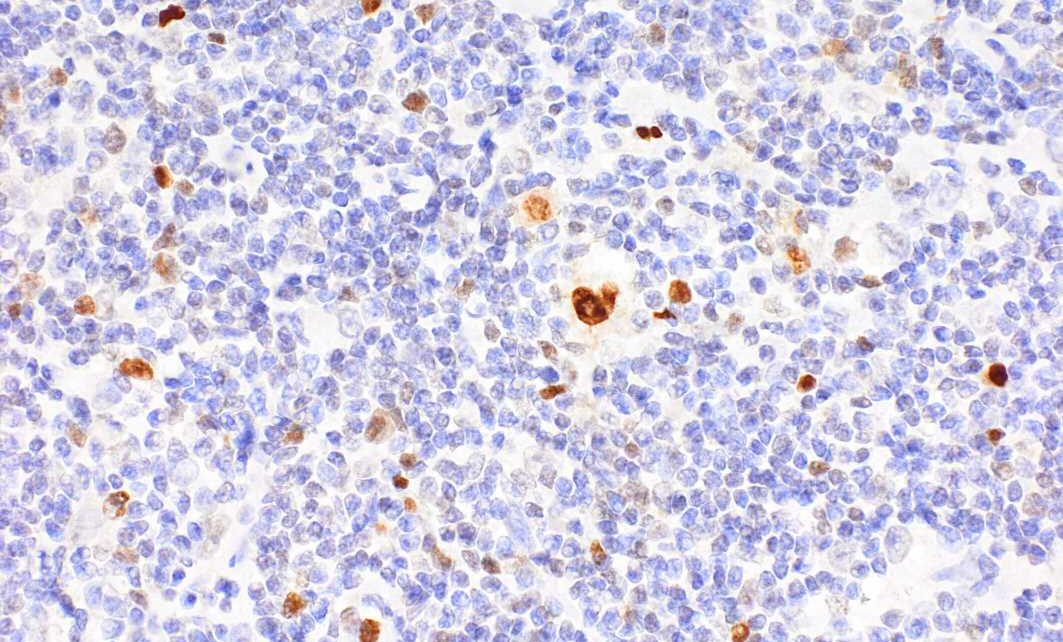
Hodgkin’s lymphoma is one of the most common types of lymphoma in young adults. It is characterized by the presence of enlarged B lymphocytes, which are unusual in that they bear on their surface the identifying markers of many other immune cells—such as those found on phagocytes, dendritic cells, or T cells. Now, a team led by Stephan Mathas from the Experimental and Clinical Research Center (ECRC) has explained how these changes take place in the cells and what impact they have. The ECRC is a joint institution of the Max Delbrück Center and Charité—Universitätsmedizin Berlin.
“Many different molecular changes have been identified in classic Hodgkin’s lymphoma, but we know little about the mutations that actively promote tumor cell development,” says Stephan Mathas, last author of the study published in Nature Communications.
Together with colleagues at the Institutes of Human Genetics of the Ulm University Medical Center (Prof. Reiner Siebert) and of the Polish Academy of Sciences in Poznan (Dr. Maciej Giefing), Mathas’s team trawled through vast amounts of gene sequencing and expression data in search of activating mutations. In cell lines and later in patient tumor cells, they repeatedly found the same mutation in the transcription factor IRF4 in about 15 percent of patients.
Completely changed functionality
“In these cases, within IRF4 only one amino acid has been altered: IRF4 contains an arginine instead of a cysteine at position 99,” explains Mathas. “In collaboration mainly with Pierre Cauchy and Constanze Bonifer from Birmingham in the United Kingdom, we showed that this completely changes how the transcription factor functions: It can no longer bind to its usual DNA binding sites, but instead binds to other DNA motifs that are not normally recognized. This fundamentally modifies the regulation of gene activity.” As a consequence, B lymphocytes are prevented from maturing into antibody-producing plasma cells.
Since this mutation is common in Hodgkin’s patients, the researchers suspected that it may be advantageous to tumor cells. So they decided to generate healthy B cells capable of producing both the “normal” and altered IRF4 and then to analyze which genes are regulated by the mutant. When the IRF4-C99R mutant was turned on, the healthy plasma cell program remained silent. Instead, it resulted in the upregulation of genes commonly associated with Hodgkin’s lymphoma—genes that in their healthy state cannot be turned on at all by IRF4.
Irregular docking activity
The comparatively bulky arginine of the IRF4-C99R mutant no longer fits into the usual DNA docking sites, but it does bind well to other sites on the DNA. This results in the activation of disease-relevant genes.
“A key finding for us was that a point mutation can lead to both a loss and an increase in DNA binding ability, and thus has fundamental consequences for gene function,” says Dr. Nikolai Schleussner, co-first author of the study along with Pierre Cauchy. Such changes at DNA binding sites are difficult to predict using bioinformatics techniques. That is why, says Schleussner, it was so important to combine experimental work with innovative bioinformatics analyses by researchers in Birmingham, Vancouver, and at MDC-BIMSB in Berlin.
A previous study by Mathas’s team and international collaborators, published in January 2023 in Science Immunology, had already shown that changing a single amino acid in this transcription factor can have big consequences. During that research, the scientists discovered an IRF4 mutation located only a few amino acids away that causes a severe, previously unknown immunodeficiency in children. “We believe we have described a mechanism that also plays a key role in other diseases,” stresses Mathas.
The current study suggests it may be possible to block the misdirected activation of a transcription factor.
“Today it is difficult to directly block transcription factors. But now that we know where the mutant binding sites are, we can look for inhibitors that occupy precisely these DNA binding pockets while leaving healthy cells untouched,” says Mathas.
More information:
Nikolai Schleussner et al, Transcriptional reprogramming by mutated IRF4 in lymphoma, Nature Communications (2023). DOI: 10.1038/s41467-023-41954-8
Journal information:
Science Immunology
,
Nature Communications
Source: Read Full Article