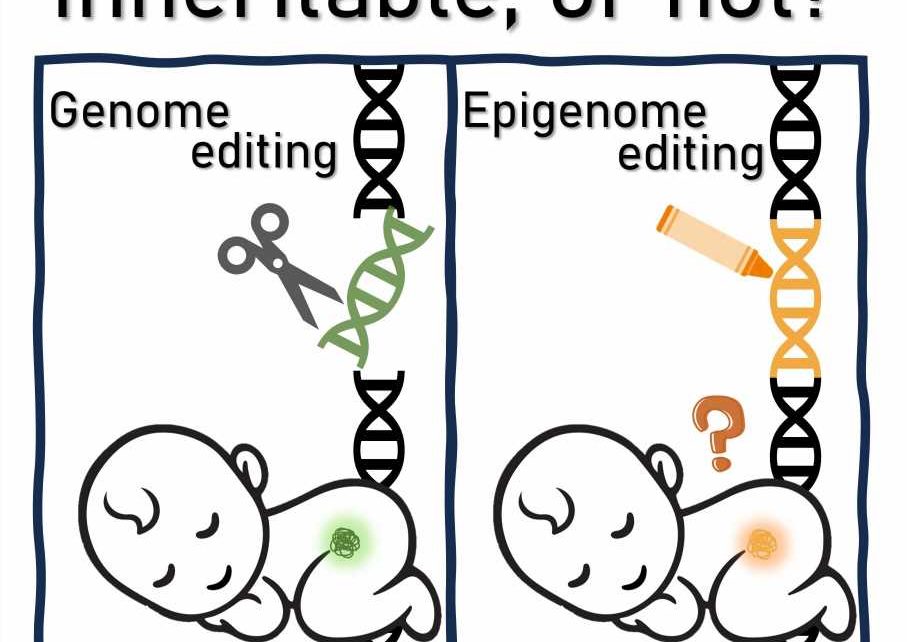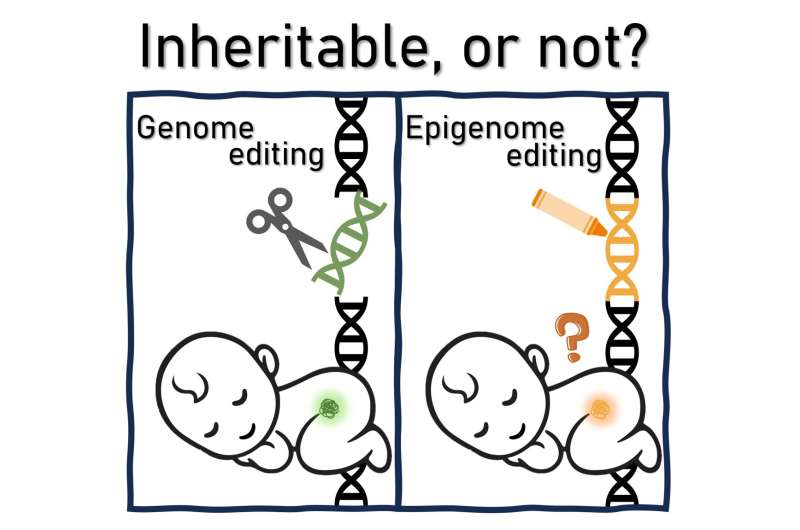
Epigenome editing is an emerging technology used to regulate gene function by controlling epigenetic states at specific locations on the genome. This method is distinct from traditional genetic editing, which involves permanently altering the DNA sequence. Notably, the intervention effects of epigenome editing are thought to be reversible, making this technology particularly attractive for its potential therapeutic applications in the treatment of genetic disorders and chronic diseases.
Although some researchers argue that it presents fewer ethical issues compared with permanent genome editing, especially in terms of its impact on offspring, the potential for transgenerational epigenetic inheritance has also been reported, suggesting that epigenetic changes could be inherited across generations in mammals.
A new study published in Stem Cell Reports sought to examine the ethical and practical questions of epigenome editing and its use for therapeutic purposes, especially in the context of transgenerational epigenetic inheritance and the potential consequences for future generations.
The epigenome comprises chemical modifications to the genome that regulate gene expression. Epigenome editing holds much promise for treating genetic disorders and chronic diseases. It aims to adjust genome function, primarily gene expression, without altering the DNA sequence, and involves various molecular mechanisms, including DNA methylation, histone modifications, non-coding RNAs, chromatin structure, and transcription factor binding. However, disruptions in these mechanisms can lead to genetic disorders and cancers.
This study discusses two categories of epigenome editing: “on-site-only” editing, which has temporary effects that are relatively easy to halt, and “memory-forming” editing, which induces persistent epigenetic changes even after the editing tool is removed. This second category in particular presents challenges related to potential irreversibility, similar to genome editing.
The germline—the genetic material passed on to offspring—is of particular concern for transgenerational inheritance. While germline applications of epigenome editing have not yet been reported, the paper discusses theoretical scenarios where it could affect germ cell lineages, such as in prenatal fetuses and postnatal infants, and draws parallels with the ethical concerns associated with heritable genome editing—especially in the editing of human embryos for clinical purposes.
Other concerns are potential off-target effects in epigenome editing, which can result in unintended changes across the genome. These off-target effects may have a lasting impact, especially in cases of memory-forming epigenome editing. Furthermore, the outcome of epigenetic effects may be more challenging to assess than genetic changes.
While transgenerational epigenetic inheritance has been observed in organisms such as yeast, plants, and nematodes, it remains unclear whether this phenomenon occurs in mammals. Importantly, epigenetic changes can also occur in response to environmental factors and can be passed on across generations in some organisms.
The authors argue that if this true, it may be premature to claim that epigenome editing inherently poses fewer ethical issues than traditional genome editing, and thus there is a need for more comprehensive discussion on the ethics and regulation of clinical applications of epigenome editing in humans.
Considering the possibility of transgenerational epigenetic inheritance, the authors contend that discussions about the ethics and regulation of epigenome editing should not be limited to considering it as a non-inheritable technology. Each application of epigenome editing should be carefully evaluated, considering the specific epigenetic effects employed, their persistence, and the method of delivery. However, assessing the safety of these applications and their effects on future generations is difficult.
To address these challenges, the authors propose three criteria for evaluating the validity of human applications of epigenome editing for disease treatment: selectivity of the intervention for target tissues or cells, the possibility of direct effects on the germline, and the severity of disease symptoms.
While inheritable epigenomic interventions may appear appealing for preventing disease transmission to offspring, they raise enduring ethical concerns regarding their effects on future generations, much like heritable genome editing. Therefore, the authors advocate for establishing stringent regulations similar to those governing genome editing and gene therapy, particularly for future inheritable epigenome editing and embryonic interventions. This recommendation underscores the need for careful management of epigenome editing to ensure ethical and safe applications in clinical fields.
More information:
Mitsuru Sasaki-Honda et al, Is epigenome editing non-inheritable? Implications for ethics and the regulation of human applications, Stem Cell Reports (2023). DOI: 10.1016/j.stemcr.2023.10.003
Journal information:
Stem Cell Reports
Source: Read Full Article