A new test revealed that FDA-approved antibiotics—available at your neighborhood pharmacy—can effectively treat superbugs. They are not prescribed, however, because the gold-standard test predicts they will not work. The new test may improve the way antibiotics are developed, tested and prescribed—and it is openly available to all.
The research has significant implications in the fight against bacterial resistance by optimizing the prescription and use of currently available antibiotics and enhancing the efforts to discover new ones.
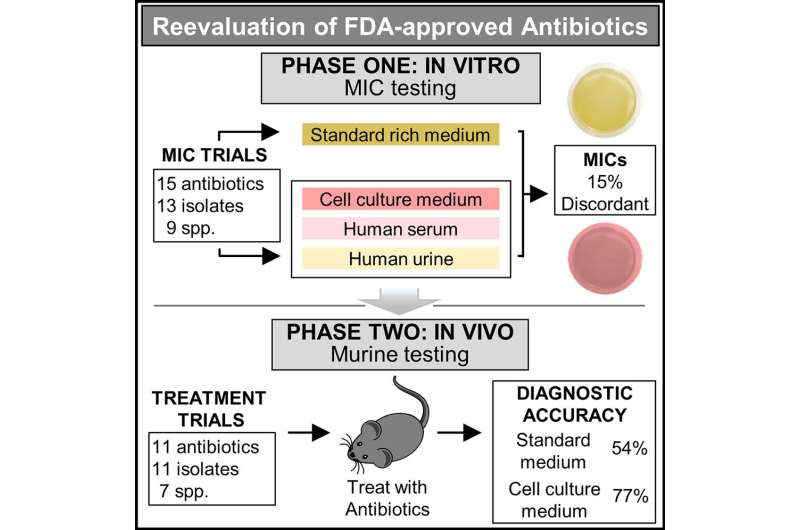
Developed by a research team of UC Santa Barbara scientists, the antibiotic study was published in the journal Cell Reports Medicine. The research addressed a fundamental flaw in the healthcare paradigm for determining antibiotic resistance. It does not account for environmental conditions in the body that impact drug potency.
By simulating conditions in the body, the new test identified several effective antibiotics rejected by standard testing. Further, when the new and standard tests agreed—a nearly perfect prediction of treatment success or failure was observed.
The study required a tour de force screening of more than 500 antibiotic-bacteria combinations. The findings suggest that the standard test is incorrect ~15% of the time. And since physicians rely on this test for treatment decisions—it may lead to prescription of the wrong antibiotic.
The project was led by professor Michael Mahan and his UC Santa Barbara research team of Douglas Heithoff, Lucien Barnes and Scott Mahan, along with Santa Barbara Cottage Hospital physicians Lynn Fitzgibbons, M.D. and Jeffrey Fried, M.D., and professor John House of University of Sydney, Australia.
“People are not Petri plates—that is why antibiotics fail,” said Mahan. “Testing under conditions that mimic the body improves the accuracy by which lab tests predict drug potency.”
Physicians are aware of the flaws in the gold-standard test. When recommended antibiotics do not work, they must rely on their experience to decide on the appropriate antibiotic(s) for their patients.
This study provides a potential solution to address the disparity between antibiotics indicated by standard testing and actual patient outcomes.
“Reevaluation of FDA-approved antibiotics may be of far greater benefit than the time and cost of developing new drugs to combat antimicrobial resistance,” explained Fitzgibbons, an infectious disease physician, “potentially leading to significant life-savings and cost-savings.”
“Sepsis treatments are expensive and require long hospital stays,” explained Heithoff, “and testing and re-testing is not only time- and labor-intensive, but also leads to antibiotic resistance.”
The new test will lead to reduced costs for the healthcare industry in their efforts to identify new drugs to fight antimicrobial resistant infections.
“More accurate testing reduces the costs of drug discovery by streamlining detection of lead candidates long before expensive human clinical trials,” said House, a clinical veterinarian.
Added Fried, a critical care physician, “Human clinical safety and efficacy studies will need to be conducted to assure these findings are applicable to patients with various infections and sepsis.”
“As a Gaucho, I’m always proud to advance legislation that delivers critical support for the great work that UCSB ICB and other researchers are doing,” said Rep. Salud Carbajal.
“With the support provided from the laws created by my colleagues and I on the Armed Services Committee, UCSB ICB was able to develop a new test method which revealed that FDA-approved antibiotics can effectively treat multidrug-resistant superbugs. This would be a game changer for many in our community with limited access to health care, and I’m proud to see the support included in the legislation I helped get signed into law play a part in this breakthrough.”
More information:
Douglas M. Heithoff et al, Re-evaluation of FDA-approved antibiotics with increased diagnostic accuracy for assessment of antimicrobial resistance, Cell Reports Medicine (2023). DOI: 10.1016/j.xcrm.2023.101023
Journal information:
Cell Reports Medicine
Source: Read Full Article



