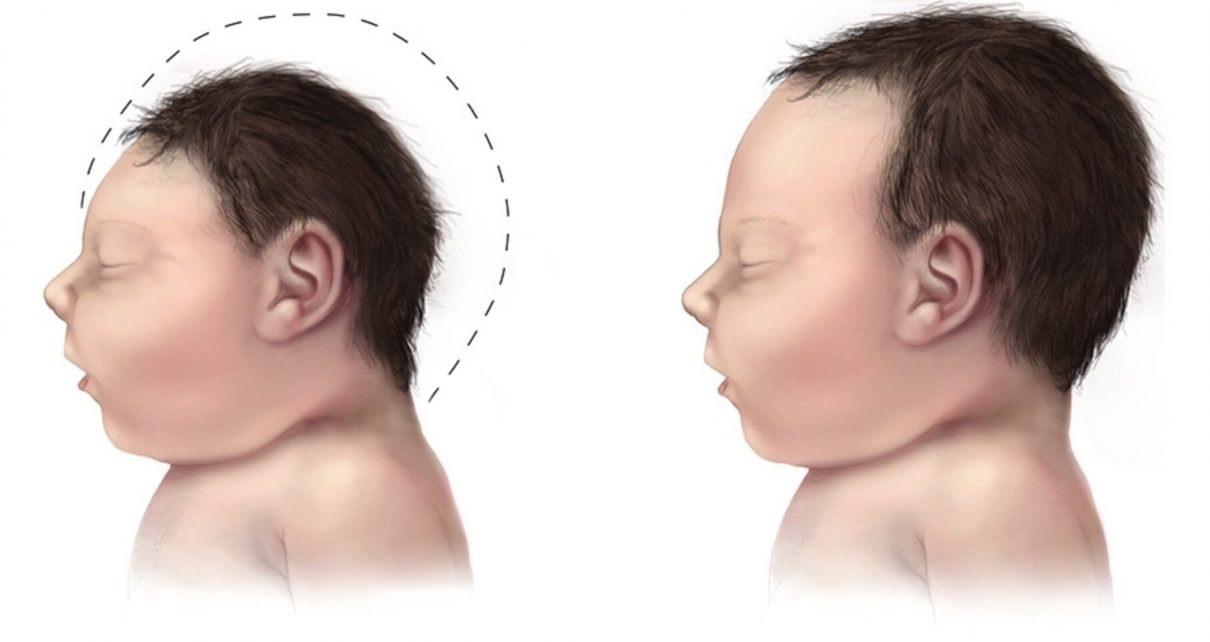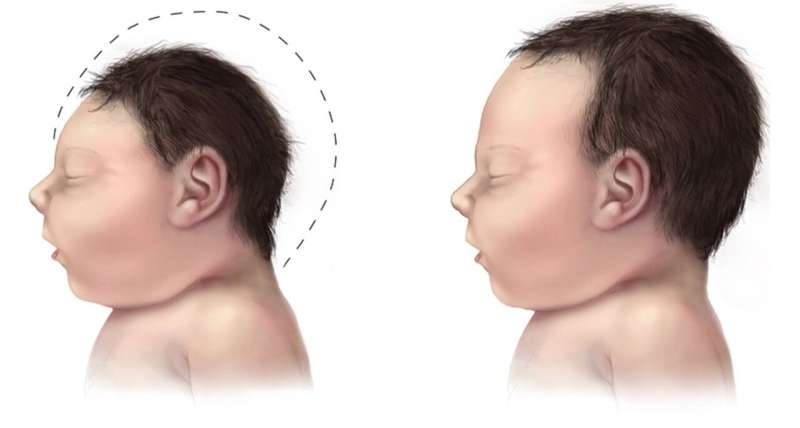
Researchers in Brazil affiliated with the State University of Campinas (UNICAMP), D’Or Institute and the Federal University of Rio de Janeiro (UFRJ) have identified molecular processes that may help explain microcephaly in babies born to mothers infected by the Zika virus. The research proposes a model at the molecular level to understand the complications caused by infection during pregnancy and paves the way to the identification of novel therapeutic targets.
According to an article on the study published in Molecular Neurobiology, the researchers conducted a proteomic analysis to detect protein expression alterations in infected cells.
They discovered that when Zika virus invades the fetal brain, it modulates energy production and controls the RNA metabolism expressed in cell nuclei. In the proposed model, these alterations interfere with the maturing of particles that act as precursors of oligodendrocytes—neural cells that produce myelin, a lipidic substance of vital importance to the exchange of information between neurons.
“When oligodendrocytes fail to mature properly, myelin sheath formation may be defective, and this has very adverse consequences for brain development,” said Daniel Martins-de-Souza, last author of the article and a professor at the State University of Campinas’s Institute of Biology (IB-UNICAMP).
“Normally, viruses infect cells in order to multiply freely and then advance to other parts of the host organism. In the case of the Brazilian strain of Zika, we observed more alterations in proteins associated with the metabolism when the virus invaded neural cells, rather than more alterations in the expression of proteins associated with these classic finalities,” Martins-de-Souza said.
To reach these conclusions, the researchers conducted two different kinds of experiment. First, they infected human neural stem cells with the Brazilian strain of Zika and analyzed the changes in protein expression. The human neural stem cells were obtained from induced pluripotent stem cells (iPSCs)—skin cells reprogrammed to develop into neural stem cells.
After the experiment with stem cells, the researchers used infected neurospheres (organoids cultured in the laboratory to simulate the morphology and functioning of part of the brain) to observe what may happen during neurodevelopment.
To compare the results, they repeated the experiments on neural stem cells and neurospheres infected by dengue and the African strain of Zika, neither of which normally infect brain cells or cause microcephaly.
“Experiments using dengue and African Zika don’t reflect what happens in nature, because these viruses don’t cross the blood-brain barrier, which protects the brain against invading pathogens. However, they enabled us to analyze and compare their proteomics and also to understand what Brazilian Zika triggers in neural cells,” Martins-de-Souza said.
In the experiments involving neural stem cells, Brazilian Zika behaved very differently from the other two viruses. “Whereas infection by dengue virus and African Zika virus was associated with increased production of proteins linked to cell domination and replication, the Brazilian strain modulated this highly important part of neural development, influencing the differentiation of neurons and glial cells [astrocytes, microglia and oligodendrocytes],” he said.
They also behaved differently in the neurospheres. “Again, Brazilian Zika modulated the cellular metabolism and also controlled the metabolism of the RNA [expressed in the nuclei of infected cells]. Both are important to explain microcephaly,” he said.
Myelin sheath
According to Martins-de-Souza, viruses have various effects when they boost or inhibit the expression of proteins linked to human metabolism. In the case of Brazilian Zika, the virus impaired neural cell maturation and early brain development by altering the expression of a protein family called hnRNP (heterogeneous nuclear ribonucleoproteins).
“These proteins are essential to the development of oligodendrocytes and myelin sheath production. Brazilian Zika appears to interfere with this process and can lead to defective myelination even before the formation of neuronal cells in the fetus,” he said.
The myelin sheath is like the insulation around an electrical wire, he explained. “It protects the ‘circuits’ in the brain,” he said. Neurons are connected both by electrical pulses and chemically. “In the brain, the myelin sheath protects the axons. If oligodendrocytes and the myelin sheath aren’t there to protect the neurons, the energy is lost.”
Axons are portions of neurons that carry nerve impulses to other neurons or muscle or gland cells. Oligodendrocytes are primarily responsible for forming and maintaining the myelin sheath that surrounds axons. They are present in the fetus and important to its neurodevelopment.
Source: Read Full Article