A recent study by scientists at Celentyx Ltd, the University of Birmingham, and the University of Warwick and posted to the bioRxiv* preprint server illustrated a cell-based severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S) protein attachment assay.
 Study: A cell-based, spike protein binding assay highlights differences in antibody neutralising capacity for SARS-CoV-2 variants. Image Credit: Design_Cells / Shutterstock
Study: A cell-based, spike protein binding assay highlights differences in antibody neutralising capacity for SARS-CoV-2 variants. Image Credit: Design_Cells / Shutterstock
Background
A crucial step for SARS-CoV-2 entrance into human cells is the attachment of the SARS-CoV-2 S protein with the angiotensin-converting enzyme 2 (ACE2) receptor. Therefore, preventing this engagement is a significant determinant in the therapeutic effectiveness of serum antibodies induced by the coronavirus disease 2019 (COVID-19) vaccination and therapeutic monoclonal antibodies.
Moreover, the appearance of SARS-CoV-2 variants like Omicron and Delta has made it necessary to develop flexible assays that could assess the efficacy of COVID-19 therapeutics. Arising SARS-CoV-2 variants might show resistance to or evasion from neutralization by COVID-19 monoclonal antibodies or antibodies produced by vaccination centered on the initial SARS-CoV-2 strain. Indeed, understanding these constraints guides political and health strategies during the societal challenges brought on by the COVID-19 pandemic.
About the study
The goal of the present study was to establish a reductionist cell-based framework suited for high-throughput assessment that measures inhibition of SARS-CoV-2 S protein adherence to mammalian cells appropriate to aid immune tracking and development of therapeutics.
Initially, the team explored the attachment of a recombinant His-tagged SARS-CoV-2 S protein (S A) to lung epithelial A549 cell lines, either over-expressing ACE2 (A549-ACE2), wild-type (A549-WT), or over-expressing ACE2 and transmembrane protease, serine 2 (TMPRSS2) (A549-ACE2-TMPRSS2). The His-tagged S protein was evaluated utilizing a mouse immunoglobulin G (IgG) anti-His antibody, a secondary anti-mouse IgG antibody coupled to Alexa Fluor 488 (AF488) dye, and high-content imaging measuring the intensity of S protein labeling per cell. Subsequently, the attachment of several recombinant S proteins to A549-ACE2 and A549-WT cells was analyzed.
The effects of various widely available anti-S antibodies on the labeling of ACE2-A549 cells by S A and S F were evaluated to examine the effectiveness of the currently designed assay for monoclonal antibody testing. Further investigations were proceeded using three antibodies. The efficacy of receptor-binding domain 8 (RBD8), RBD1, and RBD7 antibodies to neutralize SARS-CoV-2 infection of Vero cells was explored to gauge the translational significance of the assay.
The performance of the S binding assay in plasma from individuals was analyzed with anti-S IgM/G/A measured by enzyme-linked immunosorbent assay (ELISA) to increase the potential utility of this assay. In addition, a score ≥ 1.0 was regarded as positive in this evaluation. Besides, the performance of RBD8, RBD14, and RBD7 was compared against the SARS-CoV-2 Omicron and Delta S protein labeling of A549-ACE2 cells.
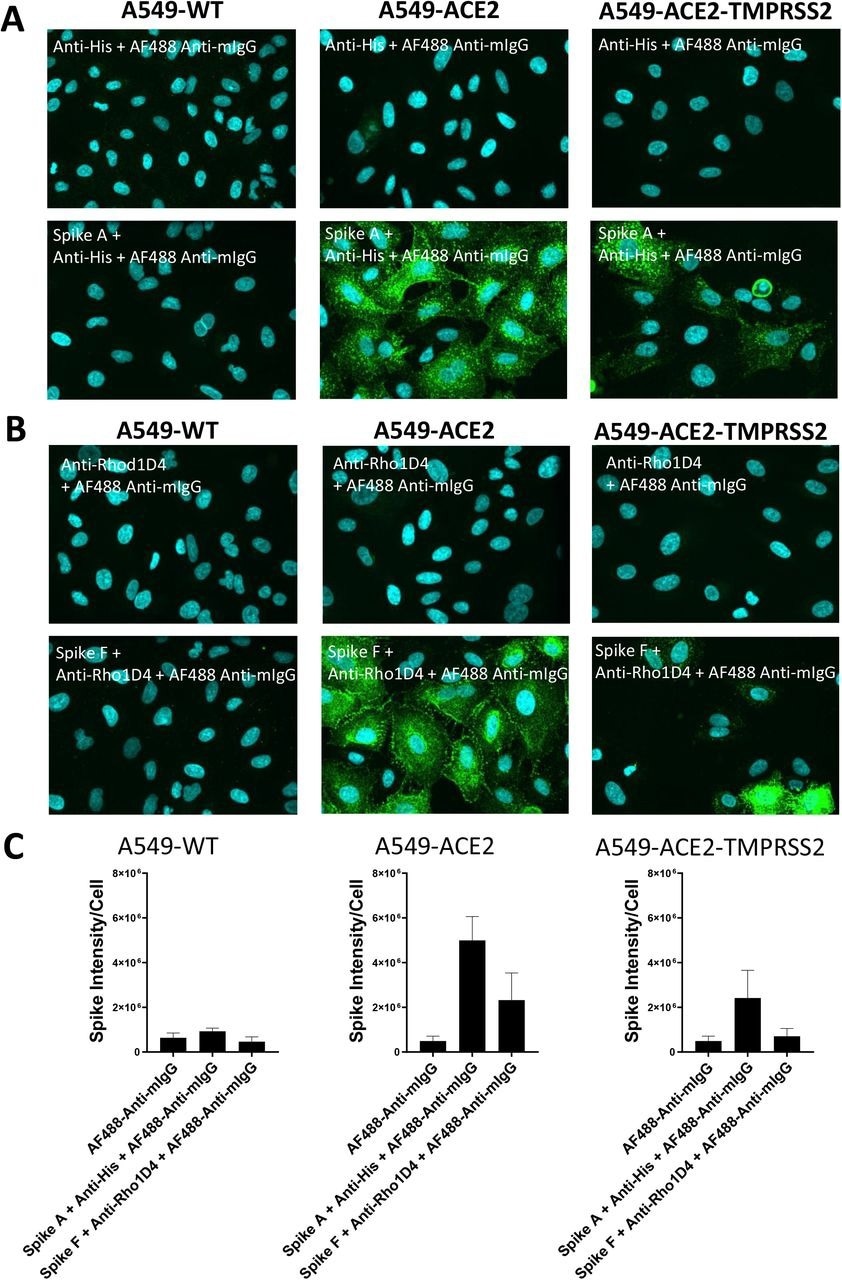
Labeling of ACE2 expressing A549 cells by recombinant spike proteins (A) Representative confocal images showing labeling of wild type (A549-WT), ACE2-overexpressing (A549-ACE2) and ACE2 and TMPRSS2-overexpressing (A549-ACE2-TMPRSS2) A549 cells by recombinant spike protein A (‘Spike A’) and anti-His + AF488-conjugated anti-mIgG detection antibodies. (B) Representative confocal images showing labeling of A549 cells (A549-WT), ACE2-overexpressing (A549-ACE2) and ACE2 and TMPRSS2-overexpressing (A549-ACE2-TMPRSS2) A549 cells by recombinant spike protein F (‘Spike F’) and anti-His + AF488-conjugated anti-mIgG detection antibodies (green; nuclei in cyan). (C) Quantification of spike protein labeling intensity from images acquired by high content confocal microscopy for cells labeled in (A) and (B). Data expressed as mean + SEM from three independent experiments for A549-ACE2 and from two independent experiments for A549-WT.
Results and conclusions
The study results showed that while A549-WT cell labeling with S protein was similar to background concentrations, both A549 cells overexpressing ACE2 and TMPRSS2 and those overexpressing ACE2 had punctate labeling of S protein. The S protein labeling was less intense on A549-ACE2-TMPRSS2 cells than on A549-ACE2, which might be due to different levels of ACE2 expression in the cell lines. An anti-mouse IgG secondary antibody coupled to AF488 and an anti-RhoD1A4 antibody showed clear labeling upon subsequent use of an S protein inside a detergent micelle (S F), which presumably comprises the trimeric S in its native structure.
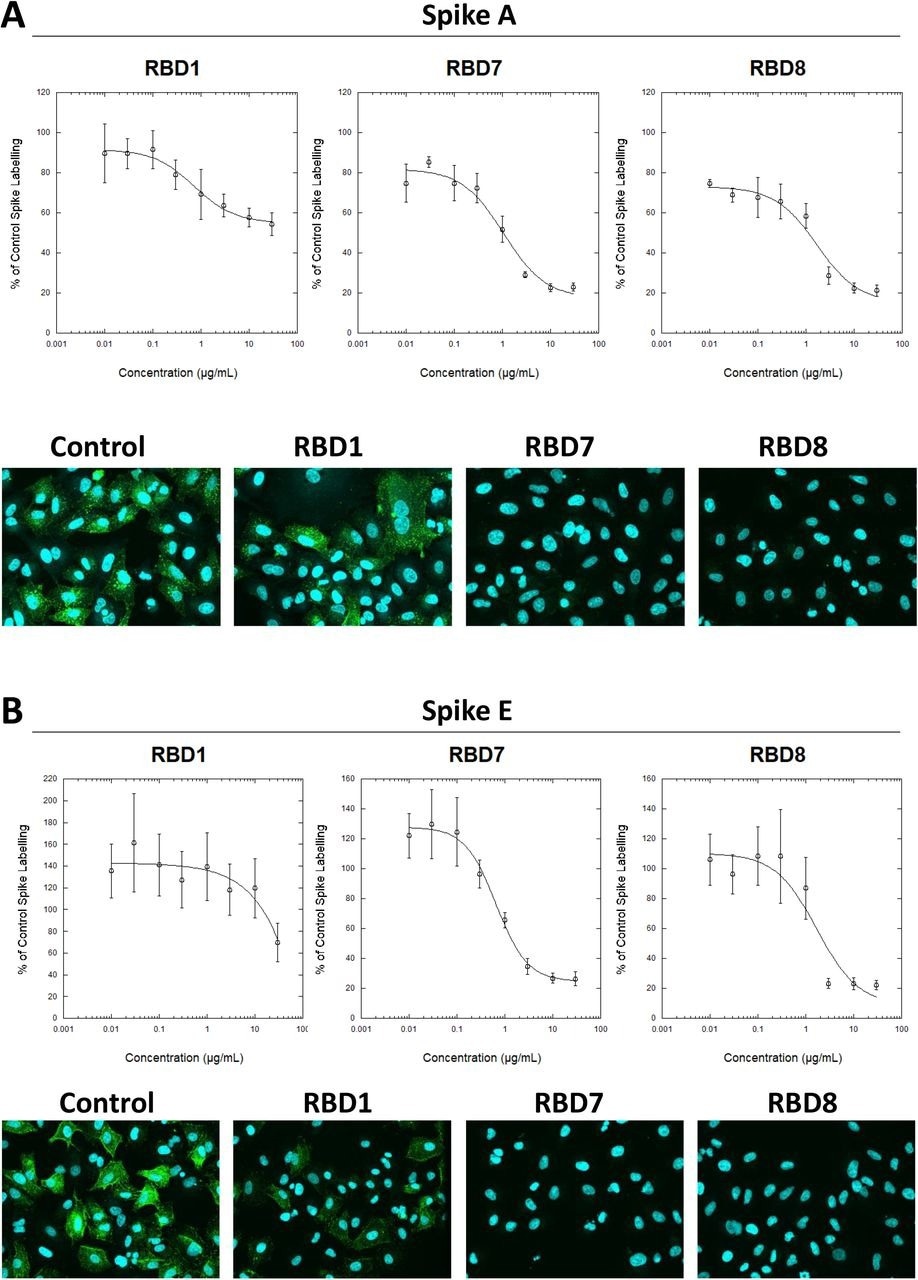
Characterization of spike blocking antibodies Labelling of A549-ACE2 cells by recombinant spike protein A (A) or E (B) that had been pre-incubated in the absence or presence of the indicated concentrations of antibodies RBD1, RBD7 or RBD8, followed by detection using anti-His + AF488-conjugated anti-mouse IgG antibodies, and visualization and quantification of spike protein labeling intensity by confocal microscopy. Plots show data expressed as mean ± SEM of three independent experiments. A solid line represents nonlinear regression using a 4-parameter logistic equation. Representative confocal images show labeling of cells under control conditions or with spike protein pre-incubated with 10 μg/mL RBD1, RBD7 or RBD8.
Subsequently, the analysis of various recombinant S proteins in A549-ACE2 and A549-WT cells displayed that adherence to A549-WT was typically poor. Nevertheless, different labeling intensities were visible in A549-ACE2 cells, with some recombinant S proteins showing a weak signal. These results demonstrated that some recombinant S proteins permitted clear labeling of A549-ACE2 cells.
The labeling of both S A and S F was decreased consistently by antibodies RBD5, RBD1, RBD8, and RBD7. Titration of RBD7, RBD1 and RBD8 antibodies demonstrated concentration-reliant blockage of S protein A and E labeling, enabling quantification of each antibody's half-maximal inhibitory concentration (IC50) values. RBD1 had the lowest strength to neutralize viral infection, mirroring the S labeling results with S A; however, RBD8 and RBD7 successfully stopped the viral infection, with RBD8 having higher potency than in the S binding experiment. Additionally, human plasma samples with anti-S IgA/M/G levels above three showed concentration-dependent S blocking action; on the other hand, those with anti-S IgA/M/G levels less than three did not. These findings imply that the present S binding assay could characterize monoclonal antibodies and track neutralizing antibody function in plasma.
Furthermore, the authors discovered that while RBD14, RBD7, and RBD8 significantly decreased labeling by the Delta S protein, just RBD14 showed a similar efficiency against the Omicron S protein.
In summary, the team has created a high-content imaging technique to measure SARS-CoV-2 S protein attachment to target cells expressing ACE2 in the current investigation. The investigators envisage that the cell-based S protein assay established in this research would supplement existing pseudovirus and biochemical assays in COVID-19 therapeutic development. It could also be modified to work with other cell kinds to explore the potentially ACE2-autonomous adherence of the SARS-CoV-2 S protein.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- A cell-based, spike protein binding assay highlights differences in antibody neutralising capacity for SARS-CoV-2 variants; Neale Harrison, Lauren Richardson, Chiara Pallini, Ines Morano, Elizabeth Jinks, Jamie Cowley, Hujo Chan, Harriet J Hill, Cristina Matas de las Heras, Ana Teodosio, Andrea S Lavado, Timothy R Dafforn, Dimitris K Grammatopoulos, John Gordon, Catherine A Brady, Lawrence S Young, Nicholas M Barnes, Zania Stamataki, Omar S Qureshi. bioRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.06.24.496409, https://www.biorxiv.org/content/10.1101/2022.06.24.496409v1
Posted in: Device / Technology News | Medical Science News | Medical Research News | Disease/Infection News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Antibodies, Antibody, Assay, Cell, Cell Labeling, Confocal microscopy, Coronavirus, Coronavirus Disease COVID-19, covid-19, Efficacy, Enzyme, Imaging, Immunoglobulin, Mammalian Cells, Microscopy, Monoclonal Antibody, Omicron, Pandemic, Protein, Protein Labeling, Pseudovirus, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Secondary Antibody, Serine, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Therapeutics, Titration

Written by
Shanet Susan Alex
Shanet Susan Alex, a medical writer, based in Kerala, India, is a Doctor of Pharmacy graduate from Kerala University of Health Sciences. Her academic background is in clinical pharmacy and research, and she is passionate about medical writing. Shanet has published papers in the International Journal of Medical Science and Current Research (IJMSCR), the International Journal of Pharmacy (IJP), and the International Journal of Medical Science and Applied Research (IJMSAR). Apart from work, she enjoys listening to music and watching movies.
Source: Read Full Article



