 Thought LeadersNeil Lagali and Mehrdad RafatProfessor in Experimental Ophthalmology and Adj. Associate Professor/CEO Linköping University/LinkoCare Ltd.
Thought LeadersNeil Lagali and Mehrdad RafatProfessor in Experimental Ophthalmology and Adj. Associate Professor/CEO Linköping University/LinkoCare Ltd.With corneal blindness affecting an estimated 12.7 million people worldwide, novel treatment approaches are required. In this interview, we speak to Professors Neil Lagali and Mehrdad Rafat about their latest research, which detailed a bioengineered corneal tissue for minimally invasive vision restoration in advanced keratoconus in two clinical cohorts.
Please can you introduce yourself, tell us about your scientific background, and what inspired your latest research?
Neil Lagali: I am trained as an engineer in physics and optics, but for the past 20 years, I have been performing research focusing on corneal diseases. I am currently a Professor of Experimental Ophthalmology at Linköping University in Sweden.
This research was inspired by a desire to address the large burden of global corneal blindness and the existing inequities in access to vision care. This has become even more pressing with the United Nations in 2019 adopting a resolution committing us to reach 1.1 billion people with vision impairment who lack access to eye care by 2030 as part of the Sustainable Development goals.
Mehrdad Rafat: I’m a biomedical engineer by training with a Ph.D. in Chemical & Biological Engineering from the University of Ottawa, Canada. I have more than 20 years of experience in Biomaterials and Tissue Engineering. The unmet need for corneal implants to treat corneal blindness inspired me to take on this research and development work to help people see or see better.
Corneal blindness affects an estimated 12.7 million people worldwide. What are the current treatment options and their associated challenges?
Corneal transplantation is the current gold-standard treatment option for corneal blindness. When corneal transplants fail, prosthetic devices can be used in the most severe cases, but those cases are relatively rare. For the vast majority of corneal blindness, transplantation is the only option for regaining vision.

Image Credit: Thor Balkhed/Linköping University
However, only one cornea is available for every 70 needed, and over half of the world’s population does not have access to donor corneas. Even if a donor cornea is available, the infrastructure needed to procure, store and distribute it is significant. It has to be tested for diseases and viruses, which is also expensive. Finally, after transplantation, there is always a risk of rejection of the donor tissue, meaning that immune suppressive medications must be given to patients for at least a year after transplantation.
The cornea consists mainly of the protein collagen. How was the bioengineered cornea created, and what advantages does it present over donated corneas?
We also wanted to use collagen to create a bioengineered cornea, to mimic the natural cornea. Because there is no abundant and low-cost source of human collagen, we chose to use collagen sourced from pig skin. This collagen is abundant, inexpensive, highly purified and already used in FDA-approved medical products. Briefly, the purified collagen is rehydrated and crosslinked with a non-toxic chemical crosslinker that is water soluble and washes out of the implant. Its only effect is to bind the collagen fibers to strengthen the implant. Then, in a second step, the implant, to which a small amount of riboflavin (vitamin B2) was added, is exposed to UVA light, which photochemically binds the collagen fibers further to produce a robust implant, which is a hydrogel containing almost 88% water.
The advantage of the bioengineered cornea is that it does not contain impurities, human cells, or cellular material; therefore, it has a much lower risk for rejection than donated corneas. It can also be customized to the size, thickness, and shape of the recipient. It is packaged and sterile, can be shipped anywhere in the world at room temperature, and can be stored for up to two years before use in a standard refrigerator.
Donor tissue must be procured and transplanted within two weeks and requires specialized eye banks and medicines to keep the tissue viable, which represents a high cost and infrastructure requirement, that is unfortunately not available in many developing countries. Finally, donated corneas are very scarce, whereas the bioengineered implant can, in theory, be mass produced.
You also developed a novel method for treating keratoconus. Please could you tell us about this disease, your new method, and the advantages it presents?
Keratoconus is a progressive disease starting in childhood and progressing during the teenage years. It involves a gradual breakdown of the collagen in the cornea, causing it to get weaker, thinner and lose its shape and ability to focus light. Vision gets progressively worse. The cause of keratoconus is unknown in most cases, and there is no way to prevent it.
If not detected and treated early, it will progress to a point where severe visual impairment and blindness occur, and then only a corneal transplant can restore vision. It is a common disease; however, its prevalence varies from about 0.1% of the population in the USA to up to 2-3% of the population in the Middle East, Asia, and Australia. This means that in a country such as India or China, tens of millions of people have the disease. Unfortunately, it is not detected or treated early in many countries, leading to severe vision loss and blindness.

Image Credit: Garna Zarina/Shutterstock.com
A corneal transplant is necessary for advanced stages of keratoconus when the cornea becomes too thin or irregular for preventative measures. This requires removing the entire thickness of the cornea and replacing it with a human donor cornea that is then sewn into place. Because the transplant is foreign human tissue containing cells, the patient must receive immunosuppressive eye drops for at least a year and come back to the clinic several times to adjust, replace, and remove the sutures. Even then, vision is not optimal and requires further corrective refractive procedures.
In cases where the cornea is still transparent, our method keeps the patient’s own cornea, only making a small incision within it and inserting our bioengineered implant. The implant does not have cells, so it does not trigger an immune response, and only an 8-week course of immune suppression eye drops is needed. No sutures are needed, and the procedure can be performed in a single hospital visit. The wound healing is very fast, and vision improves almost immediately. We showed that this procedure has the potential to give 20/20 vision to initially blind patients without using donor tissue. It is also a procedure that is easier to perform than standard transplantation, which we hope will allow it to be adopted in less specialized centers.
Many of those who need corneal implants live in developing countries. How important was it to you to consider economics when developing this implant?
Economics was a major consideration when we used collagen derived from pig skin as a byproduct from the food industry. This ensures a cheap, abundant, and sustainable source of collagen that will translate into low-cost corneal implants that can be mass-produced and distributed to places where the burden of corneal blindness is highest. We also considered economics when developing our implantation technique, which is simpler than current transplantation methods and does not require extensive medications or many follow-up hospital visits.
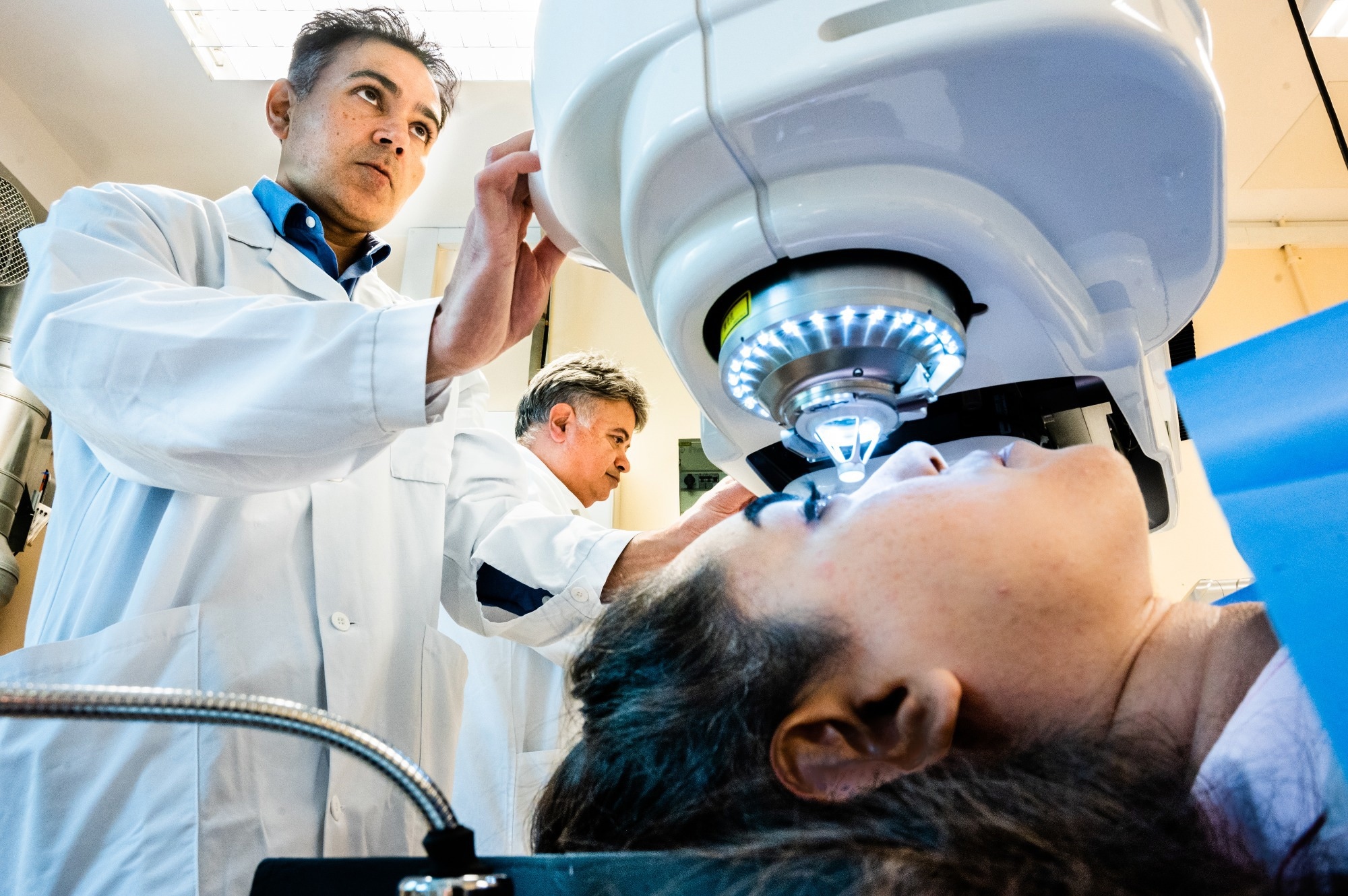
Image Credit: Thor Balkhed/Linköping University
Other eye diseases, like cataracts, cause a high incidence of blindness. Is there the possibility that similar implants could be used to treat other eye diseases?
Our work demonstrates the feasibility of implanting a biomaterial in the eye and its stability in the longer term to restore lost function. Although we haven’t tested the approach for other eye diseases, the principle has now been demonstrated. Cataracts can be treated with existing artificial implants; the challenge is to make the procedure more available globally and at a low cost. Availability and cost were key requirements that led us to develop the bioengineered corneal tissue and implantation method we now report.
How do you foresee biomaterials influencing healthcare and medicine in the future?
Biomaterials can potentially restore lost function through replacement or partial or full regeneration of tissues in the body. They can be combined with cells or drugs to achieve maximal therapeutic effects. Besides providing a large supply of well-defined tissues for transplantation without the need for donor tissue, we also foresee that biomaterials will be part of the arsenal in treating disease in the future when combined with appropriate medical knowledge, techniques, and therapeutic substances. We are entering an accelerating research phase where many teams worldwide are making breakthroughs in tissue and organ repair with the help of biomaterials. We expect that the use of biomaterials in medicine will continue to grow.
What is next for yourself and your research?
We plan to conduct randomized clinical trials with a larger number of patients. So we are working on obtaining funding for that. Once we can demonstrate this works in a randomized trial, then we will apply for authorization to market the bioengineered implant as a product. In the meantime, we are performing basic and clinical research to expand the application of the material to other eye conditions.
Where can readers find more information?
- Please see the full article at: https://www.nature.com/articles/s41587-022-01408-w
- More about our research:
- http://www.linkocare.com/
- https://liu.se/en/research/cornea-research
-
https://liu.se/en/employee/neina50
- https://liu.se/en/employee/mehra64
- Vision for Everyone: accelerating action to achieve the Sustainable Development Goals, Resolution A/75/L.108 adopted 23 July 2021, https://www.un.org/en/ga/75/resolutions.shtml
About Neil Lagali
Neil Lagali is currently Professor of Experimental Ophthalmology at Linköping University, focusing on cornea and diseases of the ocular surface. The Lagali lab performs basic science in cell culture and molecular biology and evaluation of new biomaterials, biomedical devices, and experimental substances in existing and new models of eye disease. In close collaboration with ophthalmologists, his team also performs clinical studies with a focus on biomedical imaging, mechanisms of eye disease, and new surgical therapy development for eye disease. His formal education is in engineering, physics, optics, and photonics, after which he worked in the fiber optic telecommunications industry in Canada and the USA. During the past 20 years, he has been working in the field of biomedical research and ophthalmology in particular. He has led large EU-funded research consortia and networks (www.arrestblindness.eu, www.aniridia-net.eu), holds numerous patents, and has published over 100 papers in the field of cornea research. He is currently associate editor for the journals Scientific Reports and The Ocular Surface.
The Lagali lab performs basic science in cell culture and molecular biology and evaluation of new biomaterials, biomedical devices, and experimental substances in existing and new models of eye disease. In close collaboration with ophthalmologists, his team also performs clinical studies with a focus on biomedical imaging, mechanisms of eye disease, and new surgical therapy development for eye disease. His formal education is in engineering, physics, optics, and photonics, after which he worked in the fiber optic telecommunications industry in Canada and the USA. During the past 20 years, he has been working in the field of biomedical research and ophthalmology in particular. He has led large EU-funded research consortia and networks (www.arrestblindness.eu, www.aniridia-net.eu), holds numerous patents, and has published over 100 papers in the field of cornea research. He is currently associate editor for the journals Scientific Reports and The Ocular Surface.
About Mehrdad Rafat
Mehrdad Rafat is currently an Adjunct Associate Professor of Tissue Engineering at the Department of Biomedical Engineering at Linköping University in Sweden and the co-founder and CEO of Linkocare Life Sciences AB, the start-up company that has manufactured the bioengineered corneas used in the current study, and NaturaLens AB, a new start-up mandated to develop natural contact lenses for treating myopia in dry eye patients in collaboration with the European Institute of Innovation and Technology (EIT-Health).
He is an experienced entrepreneur with a demonstrated history of working in higher education, government agencies, and industry in Canada, US, and Sweden. Skilled in Biomaterials, Tissue Engineering, and Ophthalmic Medical Devices with strong educational background with a Ph.D. degree in Chem ical & Biological Engineering from the University of Ottawa, Canada, and postdoctoral fellowships at Health Canada and Ottawa Health Research Institute. I have founded successful life science spin-off companies out of my academic research including LinkoCare and NaturaLens AB.
ical & Biological Engineering from the University of Ottawa, Canada, and postdoctoral fellowships at Health Canada and Ottawa Health Research Institute. I have founded successful life science spin-off companies out of my academic research including LinkoCare and NaturaLens AB.
Posted in: Thought Leaders
Tags: Aniridia, Blindness, Cell, Cell Culture, Collagen, Contact Lenses, Cornea, Drugs, Dry Eye, Education, Eye, Eye Disease, Food, Healthcare, Hospital, Hydrogel, Imaging, Immune Response, Implants, Keratoconus, Life science, Medical Devices, Medicine, Molecular Biology, Myopia, Ophthalmology, pH, Photonics, Prosthetic, Protein, Research, Skin, Tissue Engineering, Transplant, Vision Loss, Visual Impairment, Wound, Wound Healing

Written by
Danielle Ellis
Danielle graduated with a 2:1 in Biological Sciences with Professional Training Year from Cardiff University. During her Professional Training Year, Danielle worked with registered charity the Frozen Ark Project, creating and promoting various forms of content within their brand guidelines.Danielle has a great appreciation and passion for science communication and enjoys reading non-fiction and fiction in her spare time. Her other interests include doing yoga, collecting vinyl, and visiting museums.
Source: Read Full Article



