Scientists worldwide believe that vaccination is the most effective measure to contain the ongoing coronavirus disease 2019 (COVID-19) pandemic. This pandemic has been caused by an RNA virus, namely, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which is extremely infectious and belongs to the Coronaviridae family of enveloped, positive-strand RNA viruses.
Background
Several COVID-19 vaccines have received regulatory approval or emergency use authorization (EUA) from various bodies across the world, and, subsequently, vaccination programs have been initiated in many countries. These vaccines have been developed against the SARS-CoV-2 strain that was first reported in Wuhan, China, in late 2019.
The evolution of this virus owing to mutations has resulted in many SARS-CoV-2 variants, which are classified as variants of Interest (VOI) and variants of concern (VOC). The latter are known to be more infectious, with a higher mortality rate than the original Wuhan strain. In addition, some of these variants have questioned the effectiveness of the vaccines as they can escape the vaccine-induced neutralizing antibody (nAb) responses as well as immunity gained after natural infection.
Scientists have stated that all the available vaccines can effectively reduce the rate of hospitalization and mortality against all the SARS-CoV-2 variants that are circulating. However, the vaccine-elicited neutralizing antibody (NAb) responses have been reported to wane with time. Also, increasing numbers of breakthrough infections are being reported. These reports have raised concerns in the scientific community about the possibility of the emergence of variants resistant to the existing vaccines, which may be more virulent than existing SARS-CoV-2 variants. Scientists are also apprehensive about the possibility of the emergence of novel SARS-like viruses due to spillover events that could result in a new pandemic.
Owing to these problems, there is an urgent need for nAbs and vaccines that are effective against all sarbecoviruses. Recently, several individual broadly neutralizing antibodies (bnAbs) have been directly isolated from the plasma of SARS-CoV-2 recovered patients. Antibodies are also engineered, and these are far more strain-specific. Scientists believe that the development of broadly effective coronavirus vaccines can reduce the threat of vaccines becoming ineffective against new strains.
Importance of Large Panels of bnAbs
A large panel of bnAbs would ideally offer more options for its use as prophylaxis and therapy. A range of bnAbs has proved to be extremely effective in a germline targeting approach for designing an HIV vaccine. Scientists were inspired by the robust serum nAb responses in people who recovered from natural SARS-CoV-2 infection and were later vaccinated using mRNA vaccine. They isolated and characterized bnAbs from two COVID-19 convalescent donors who were recently vaccinated. These isolated bnAbs were found to be highly potent against sarbecoviruses. This research is available on the bioRxiv* preprint server.
The Main Findings
In this study, researchers adopted a targeted donor selection strategy to isolate bnAbs to sarbecoviruses. They selected two donors for this study, who were recently vaccinated with the Moderna mRNA vaccine. The authors of this study revealed that many of the isolated bnAbs were remarkably effective in neutralizing sarbecoviruses that utilize ACE2 for viral entry. They also reported a strong binding to non-ACE2-using sarbecoviruses. Additionally, the isolated bnAbs were found to be strongly effective against SARS-CoV-2 VOC.
From the peripheral blood mononuclear cells (PBMCs) of the donors, scientists sorted CD19+CD20+IgG+IgM–B cells that could bind to both SARS-CoV-2 and SARS-CoV-1 S-proteins. Only a handful of the isolated mAbs showed detectable binding to b-HCoV and α-HCoV-derived S-proteins. Only about 31% of the mAbs showed cross-reactivity with all 12 RBDs derived from all four sarbecovirus clades. 43% mAbs neutralized all four sarbecoviruses and were categorized as bnAbs. These bnAbs, which are strongly enriched for certain germline gene features, are likely to inform pan-sarbecovirus vaccine strategies.
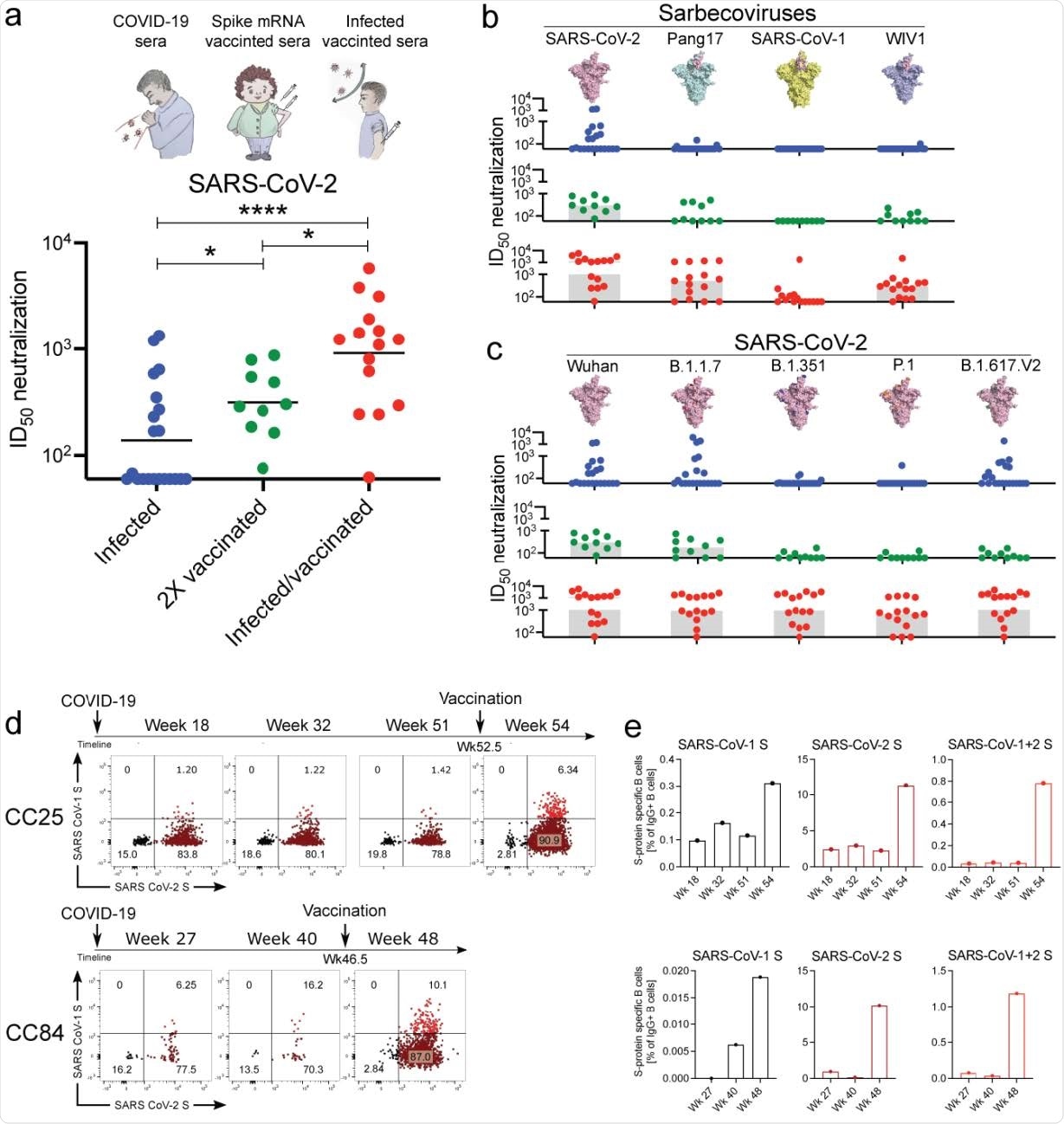
Conclusion and Future Research
In the current and future pandemics, as viral variants emerge, the availability of a wide range of potent bnAbs provides a choice of optimal reagents to scientists, for prophylaxis and therapy to respond to the viral threats. Scientists observed that certain V and D gene families were highly enriched and these genetic features could be targeted by future rational vaccine design strategies.
There are some lessons here for rational vaccine design. The higher frequency of bnAbs in infection-vaccination may be due to the fact that the S-protein may have subtle conformational differences. It could also be due to the long time lag between infection and vaccination, which might favor the accumulation of key mutations associated with bnAbs. The T cell help (by infection) may be superior to the one provided by mRNA vaccination alone. Scientists claim that there is an intriguing possibility that pan-sarbecovirus nAb activity might be best attained using a hybrid method of immunization that would seek to mimic infection-vaccination. They also opine that the bnAbs identified may themselves have prophylactic or therapeutic utility and, going ahead, will be an important contributor to rational vaccine design.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- He, W. et al. (2021). Targeted isolation of panels of diverse human broadly neutralizing antibodies against SARS-like viruses, bioRxiv, 2021.09.08.459480; doi: https://doi.org/10.1101/2021.09.08.459480, https://www.biorxiv.org/content/10.1101/2021.09.08.459480v1
Posted in: Medical Research News | Disease/Infection News
Tags: ACE2, Antibodies, Antibody, B Cell, Blood, Cell, Coronavirus, Coronavirus Disease COVID-19, Cytometric Analysis, Evolution, Frequency, Gene, Genetic, Germline, HIV, immunity, Immunization, Mortality, Pandemic, Prophylaxis, Protein, Pseudovirus, Reagents, Research, Respiratory, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine, Virus

Written by
Dr. Priyom Bose
Priyom holds a Ph.D. in Plant Biology and Biotechnology from the University of Madras, India. She is an active researcher and an experienced science writer. Priyom has also co-authored several original research articles that have been published in reputed peer-reviewed journals. She is also an avid reader and an amateur photographer.
Source: Read Full Article