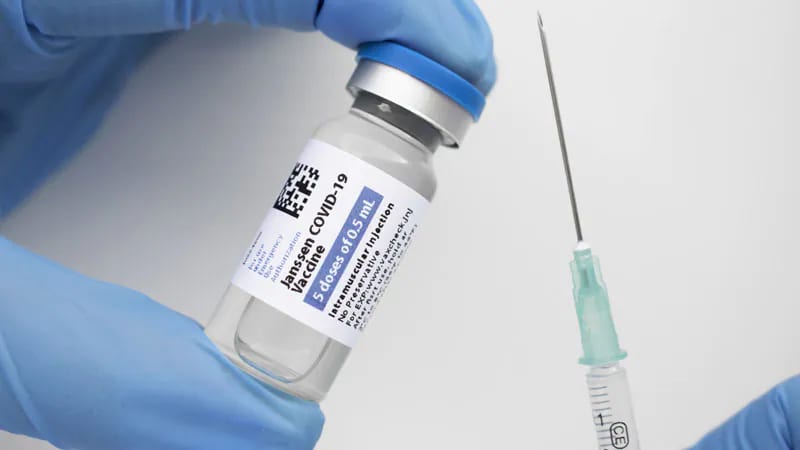
The FDA is limiting who can receive the Johnson & Johnson COVID-19 vaccine because of concerns about the risk of a rare blood clotting condition.
In a statement issued Thursday, the FDA said the J&J vaccine should only be given to people 18 and older who don’t have access to other vaccines or for whom other vaccines are not clinically appropriate. People 18 and older can also get the J&J vaccine if they choose to because they wouldn’t otherwise receive any vaccine, the FDA said.
The FDA statement was similar to the recommendation made in December by a CDC committee of experts.
The FDA said the decision was made after more information was shared about the occurrence of a rare blood clotting condition, thrombosis with thrombocytopenia syndrome (TTS), 1 or 2 weeks after people received the J&J vaccine. The finding “warrants limiting the authorized use of the vaccine,” the FDA said.
“We recognize that the Janssen COVID-19 vaccine still has a role in the current pandemic response in the United States and across the global community,” Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said in the statement.
“Our action reflects our updated analysis of the risk of TTS following administration of this vaccine and limits the use of the vaccine to certain individuals.”
The CDC says 16.9 million people are fully vaccinated with the J&J vaccine, compared to 76.5 million with Moderna and 126.3 million with Pfizer.
Through March 18, the CDC and FDA have detected 60 confirmed cases of TTS, including nine fatal cases, ABC News reported.
The J&J vaccine was granted emergency authorization in February 2021. Health authorities hoped it would help spread vaccines across the nation because it only required one initial dose and didn’t need to be stored at extremely cold temperatures, unlike the two-dose Pfizer and Moderna vaccines.
But 2 months after authorization, the government paused its use for 10 days because of reports of TTS. Last December, the CDC’s Advisory Committee on Immunization Practices said the Pfizer and Moderna vaccines were preferred over J&J because J&J carried the rare risk of blood clots and bleeding in the brain.
The FDA said the cause of the blood clotting is not known. But the “known and potential benefits of the vaccine” outweigh the risks for those people now allowed to receive it, the FDA said.
Sources:
FDA: “Coronavirus (COVID-19) Update: FDA Limits Use of Janssen COVID-19 Vaccine to Certain Individuals.”
CDC: “COVID-19 Vaccinations in the United States.”
ABC News: “FDA limits J&J COVID-19 vaccine due to rare blood clot risk.”
CDC: “Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine: Updated Interim Recommendations from the Advisory Committee on Immunization Practices — United States, December 2021.”
Source: Read Full Article