The coronavirus disease 19 (COVID-19), which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has already claimed more than 4.1 million lives worldwide.
SARS-CoV-2 is a single-stranded positive-sense RNA virus belonging to the Coronaviridae family. Other members of this family include the Middle East Respiratory Syndrome (MERS) and the severe acute respiratory coronavirus (SARS-CoV-1).
There are currently no effective antiviral drugs for the treatment of COVID-19 and vaccine rollout has been quite heterogeneous across different countries. Due to these challenges, scientists have turned to drug repurposing as a potentially promising strategy to combat the disease.
A globally available and safe drug that has properties that could ameliorate the pathological changes of COVID-19 with minimum side effects, in particular, could greatly impact the management of the current pandemic. A better understanding of the features of COVID-19 pathogenesis can also assist in drug repurposing and discovery.
To this end, a new study published in the Journal of Pharmacy and Pharmaceutical Sciences explores the use of Losartan to provide protection against COVID-19 pathogenesis.
 Study: Losartan Inhibits SARS-CoV-2 Replication in Vitro: Losartan promotes cell survival following SARS-CoV-2 infection in vitro. Image Credit: Sonis Photography / Shutterstock.com
Study: Losartan Inhibits SARS-CoV-2 Replication in Vitro: Losartan promotes cell survival following SARS-CoV-2 infection in vitro. Image Credit: Sonis Photography / Shutterstock.com
Angiotensin receptor blockers (ARBs) and COVID-19
The downregulation of angiotensin-converting enzyme 2 (ACE2) has been shown to cause local RAS dysregulation. This can subsequently lead to pro-inflammatory, pro-apoptotic, and pro-thrombotic effects and, ultimately, COVID-19-induced cytokine storm.
Scientists have hypothesized that selective AT1R antagonism by angiotensin receptor blockers (ARBs) can aid in reducing COVID-19-related lung pathology. ARBs achieve this by rebalancing the Ang II/angiotensin (1-7) ratio and by indirectly promoting Ang II-induced activation of AT2R.
Recent studies have found that C21, which is an antagonist of AT2R, improved respiratory function in the hospitalization and mortality rates of COVID-19 patients. Notably, during the initial phases of the pandemic, the use of ARBs was limited owing to concerns over their possibility to increase its viral load, due to ACE2 upregulating effects. Further research has since proven that this is not the case and experimental and clinical studies on ARBs in COVID-19 are well underway.
In recent in silico studies, Losartan, which is an ARB, was shown to change the structure of ACE2, thereby affecting its binding with the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein. Losartan was also found to reduce inflammatory responses that would otherwise lead to acute respiratory distress and change the atomic configuration of SARS-CoV-2 PLpro.
The new study
In the current study, scientists examined the ability of Losartan to inhibit the deubiquitinase and deISGylase properties of SARS-CoV-2 PLpro. The authors also examined whether Losartan was capable of preventing viral replication in pre-and post-infected Vero E6 cells.
Losartan was first incubated at various concentrations with SARS-CoV-2 PLpro and a peptide substrate, which contained interferon-stimulated gene product 15 (ISG15). The results of this experiment suggested that Losartan could be interacting with certain elements of PLpro that are capable of accommodating peptide and Ub-like substrates. The inhibition rate of Losartan was 2.3% when tested against Ub-AMC and 6.9% against ISG15 cleavage.
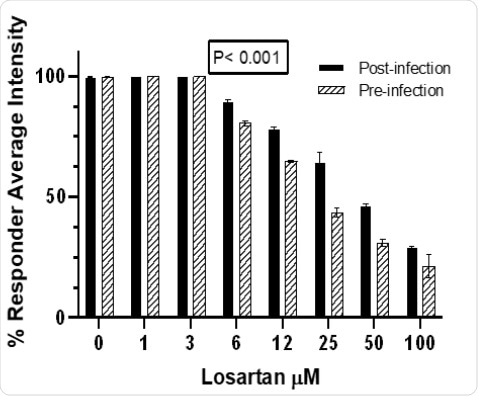
Losartan treatment, in a dose-dependent manner (1-100 μM), decreases CoV2-infected Vero E6 cells pre-infection more significantly than post-infection(Wilcoxon test p<0.001).
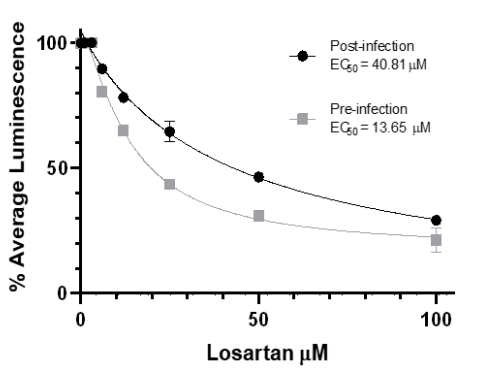
SARS-CoV-2 replication in pre-and post-infection treatment. Losartan dose-dependently reduces the production of viral nuclear proteins in Vero E6 cells (80%). EC50curves are significantly different in pre-and post-infection groups (P<0.001).
Fig. 4.Losartan treatment,in a dose–dependent manner (1–100 μM), decreases CoV2–infected Vero E6 cells pre–infection more significantlythan post–infection(Wilcoxon test p<0.001)
Fig. 4.Losartan treatment,in a dose–dependent manner (1–100 μM), decreases CoV2–infected Vero E6 cells pre–infection more significantlythan post–infection(Wilcoxon test p<0.001)
Fig. 4.Losartan treatment,in a dose–dependent manner (1–100 μM), decreases CoV2–infected Vero E6 cells pre–infection more significantlythan post–infection(Wilcoxon test p<0.001)
Losartan treatment,in a dose-dependent manner (1-100 μM), decreases CoV2-infected Vero E6 cells pre-infection more significantlythan post-infection(Wilcoxon test p<0.001).
With regard to the effect of Losartan on Tetra-Ub Deubiqutination at 2mM, Losartan showed a small reduction in deISGylase activity as compared to the control. Further, treatment of Vero E5 cells with Losartan showed a dose-dependent (0-100 µM) effect on SARS-CoV-2 replication.
Taken together, these results demonstrate the weak inhibitory effect of Losartan on viral PLpro deubiquitinase and deISGylase properties. Albeit dose-dependent, the treatment of Vero E6 cells with Losartan inhibited viral replication.
Conclusion and future research
Despite obtaining some initially promising results, more extensive research is required to gain a better understanding of the structural changes that occur in the viral proteins and their biological products when interacted with losartan. If these experiments yield viable results, they could open up new avenues for effective antiviral design and development. More research is using diversified cohorts in randomized control trials would also be necessary in order to better understand the effect of Losartan in the future stages of the pandemic.
Two of the main advantages associated with Losartan are that it is not toxic to cells and has inhibitory effects on viral replication. These features may immensely help in curbing the spread of COVID-19 and managing individuals who are unresponsive to vaccination.
There is also a possibility that Losartan is effective against future mutants of SARS-CoV-2. All of these crucial issues warrant more research. Promising results would imply easing the burden of healthcare costs globally.
- Nejat, R., Sadr, A. S., Freitas, B., et al. (2021). Losartan Inhibits SARS-CoV-2 Replication in Vitro: Losartan promotes cell survival following SARS-CoV-2 infection in vitro. Journal of Pharmacy &Amp; Pharmaceutical Sciences 24(3), pp. 390–399. doi:10.18433/jpps31931. https://journals.library.ualberta.ca/jpps/index.php/JPPS/article/view/31931
Posted in: Drug Trial News | Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Cell, Coronavirus, Coronavirus Disease COVID-19, Cytokine, Drug Repurposing, Drugs, Enzyme, Gene, Healthcare, in vitro, Losartan, Mortality, Pandemic, Pathology, Pharmacy, Protein, Receptor, Research, Respiratory, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Vaccine, Virus

Written by
Dr. Priyom Bose
Priyom holds a Ph.D. in Plant Biology and Biotechnology from the University of Madras, India. She is an active researcher and an experienced science writer. Priyom has also co-authored several original research articles that have been published in reputed peer-reviewed journals. She is also an avid reader and an amateur photographer.
Source: Read Full Article



