In a recent study posted to the bioRxiv* pre-print server, researchers demonstrated that Nitazoxanide (NTZ), an oral drug, broadly inhibits replication of several severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOCs) in preclinical models of infection.
The scientific community is toiling hard to find effective coronavirus disease 2019 (COVID-19) treatments and control the ongoing pandemic that has claimed over 5 million lives worldwide to date. An oral drug therapy that is easy to deploy, well-tolerated, inexpensive, and safe in adults and children to treat or inhibit COVID-19 progression would indeed be a significant advancement in the fight against COVID-19.
 Study: The oral drug nitazoxanide restricts SARS-CoV-2 infection and attenuates disease pathogenesis in Syrian hamsters. Image Credit: NIAID
Study: The oral drug nitazoxanide restricts SARS-CoV-2 infection and attenuates disease pathogenesis in Syrian hamsters. Image Credit: NIAID
About the study
NTZ was initially developed for the treatment of Giardia- and Cryptosporidium-associated diarrhea. In addition, in vitro studies have illustrated the ability of NTZ and its circulating metabolite tizoxanide (TIZ) to inhibit several viruses, including human coronaviruses.
In one of their previous works, the authors of the present study have demonstrated the capability of NTZ to create a broad "antiviral milieu" to overcome virus-specific immune evasion strategies. These findings prompted them to test NTZ's ability to inhibit SARS-CoV-2 replication and pathogenesis in physiologically relevant primate and human cell models.
In the present study, researchers performed antiviral assays in Vero E6 cells exposed to increasing concentrations of NTZ and TIZ and infected with SARS-CoV-2 (isolate USA-WA1/2020) at a multiplicity of infection (MOI) of 0.025 after four hours. After 48 hours, using a Celigo imaging cytometer, the 50% inhibitory concentration (IC50) of NTZ was calculated. In addition, the cytotoxicity of NTZ and TIZ was evaluated by assaying uninfected cells treated with the same compound dilutions in parallel with the antiviral assay.
Next, the researchers assessed NTZ's ability to synergize with remdesivir (RDV), standard drug treatment to shorten the time of COVID-19 recovery, for which they performed combination assays in Vero E6 cells. The results of the combination assay were further analyzed using SynergyFinder to generate a synergy landscape and combination score by the Loewe model.
Study findings
NTZ and TIZ displayed strong antiviral activity against SARS-CoV-2 in Vero E6 cells, with IC50 values of 4.04 mM and 3.62 mM, respectively, without exhibiting cytotoxicity. Fluorescence-activated cell sorting (FACS) analysis confirmed that NTZ exhibited robust antiviral activity in Vero E6 cells at both 5 and 10 mM concentrations and both MOIs tested (0.025 and 0.25).
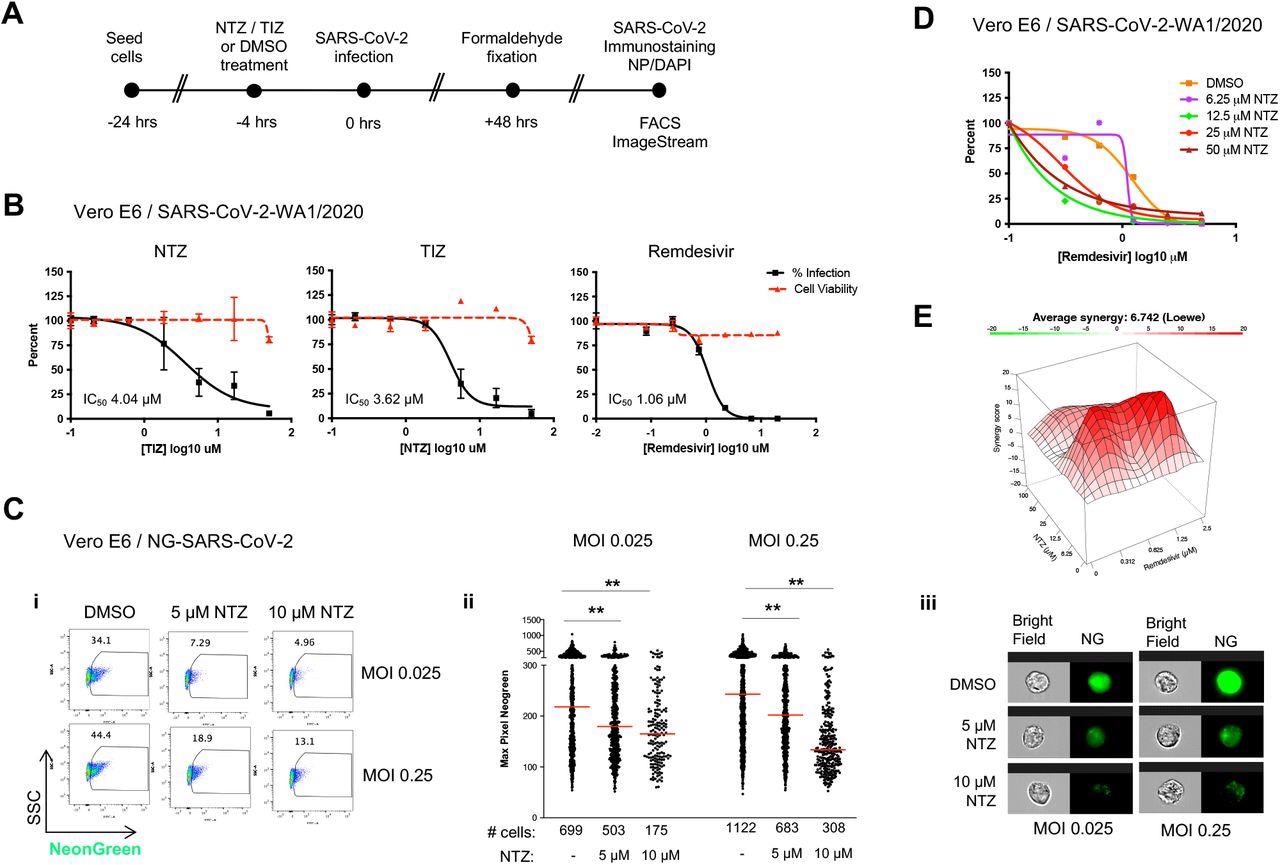
SARS-CoV-2 replication is inhibited by NTZ or TIZ in Vero E6 cells. A. Schema of the antiviral assay. B. Percent inhibition of SARS-CoV-2 replication and cytotoxicity assay in Vero E6 cells in the presence of the indicated drugs: NTZ (nitazoxanide), TIZ (tizoxanide), Remdesivir (RDV). Vero E6 cells were infected with 100 PFU (MOI 0.025) of SARS-CoV-2 (isolate USA-WA1/2020) in the presence of increasing concentrations of drug for 48 hrs, after which viral replication was measured by NP immunostaining as described in Materials and Methods. In all panels, viral infectivity is shown as a solid black line and cell toxicity as a dashed red line. Calculated IC50 is indicated in the bottom-left corner of each plot. RDV is included as a standard of care control. C. Quantitative inhibition of NeonGreen SARS-CoV-2 in Vero E6 cells measured by flow cytometry and quantitative imaging flow cytometry using the ImageStream platform. Vero E6 cells were pretreated with 5 or 10 μM NTZ or carrier (DMSO) for 4 hrs and then infected with NeonGreen (NG)-SARS-CoV2 at an MOI of 0.025 or 0.25. At 48 hrs post-infection cultures were fixed for 24 hrs before FACS and ImageStream analysis. (i) The percentage of infection as measured by FACS. Experiments were performed at least three independent times. (ii) Dot plot displaying the quantification of NG pixels as a representation of NG-SARS-CoV-2 fluorescence in infected cells (among the 3000 cells acquired). Asterisks indicate significant differences in NTZ-treated samples as compared to DMSO control by two-tailed Mann-Whitney test. **P<0.001 for each comparison. The number of infected cells in each experimental condition is shown at the bottom of the panel. We found significant reduction of infected cells in NTZ-treated cultures at both MOIs and both concentrations of NTZ (***P<0.0001 for each comparison) by Fisher exact test using Stata 12 software. Experiments were performed at least three independent times. (iii) Images of representative NeonGreen-SARS-CoV-2 infected cells were selected from the median levels of pixels shown in the analysis in panel (ii). D. Antiviral activity curves by RDV in the presence of increasing concentrations of NTZ. E. Synergy landscapes and combination scores generated by the Loewe method using SynergyFinder software.
The Loewe model shows the interaction between drugs, wherein a score greater than +10 indicates a synergistic interaction. Notably, the landscape for the interaction of RDV with NTZ showed synergy scores greater than +10 at low NTZ concentrations (6.26 mM), denoting moderate synergy of the two drugs in the inhibition of SARS-CoV-2 growth. Previous studies have shown that NTZ synergistically enhances RDV's ability to reduce the cytopathic effect (CPE). Together, this data showed that in Vero E6 cells NTZ robustly inhibited SARS-CoV-2 replication and formed an inhibitory synergistic compound pair with RDV.
NTZ (IC50 1.695 mM) and TIZ (IC50 1.322 mM) also showed potent antiviral activity against SARS-CoV-2 infection in the human cell line A549 transduced with angiotensin-converting enzyme 2 (Ace2) with no cytotoxicity.
The authors also assessed the antiviral activity of NTZ in wild-type and the type I IFN receptor (IFNAR) knock-out (KO) Ace2-A549 cells. IFNAR signaling is known to restrict SARS-CoV-2 growth and infection in Ace2-A549 cells. The results showed that although IFNAR signaling facilitates optimal NTZ inhibitory activity against SARS-CoV-2, even in its absence, NTZ inhibited SARS-CoV-2.
NTZ also strongly inhibited the replication of SARS-CoV-2 VOCs Beta 223 (B.1.351), Gamma (P.1), and Delta (B.1617.2) in Vero E6 cells, similar to its ability to inhibit SARS-CoV-2 WA1 strain.
Niclosamide, another FDA-approved drug, is structurally similar to NTZ and effectively inhibits SARS-CoV-2 spike(S)-induced syncytia formation. Interestingly, NTZ also restricted SARS-CoV-2 dissemination by directly inhibiting spike-mediated fusion, as demonstrated by the assays performed in Vero-TMPRSS2 cells.
Furthermore, NTZ-treated Syrian hamsters, compared to animals of the control group, showed strong trends of protection at four days post-infection (dpi) and six dpi. In addition, their lung biopsies showed lower viral titers at two dpi. The findings indicated that oral administration of NTZ significantly reduced SARS-CoV-2 replication, virus-induced weight loss, and overall disease pathogenesis in vivo.
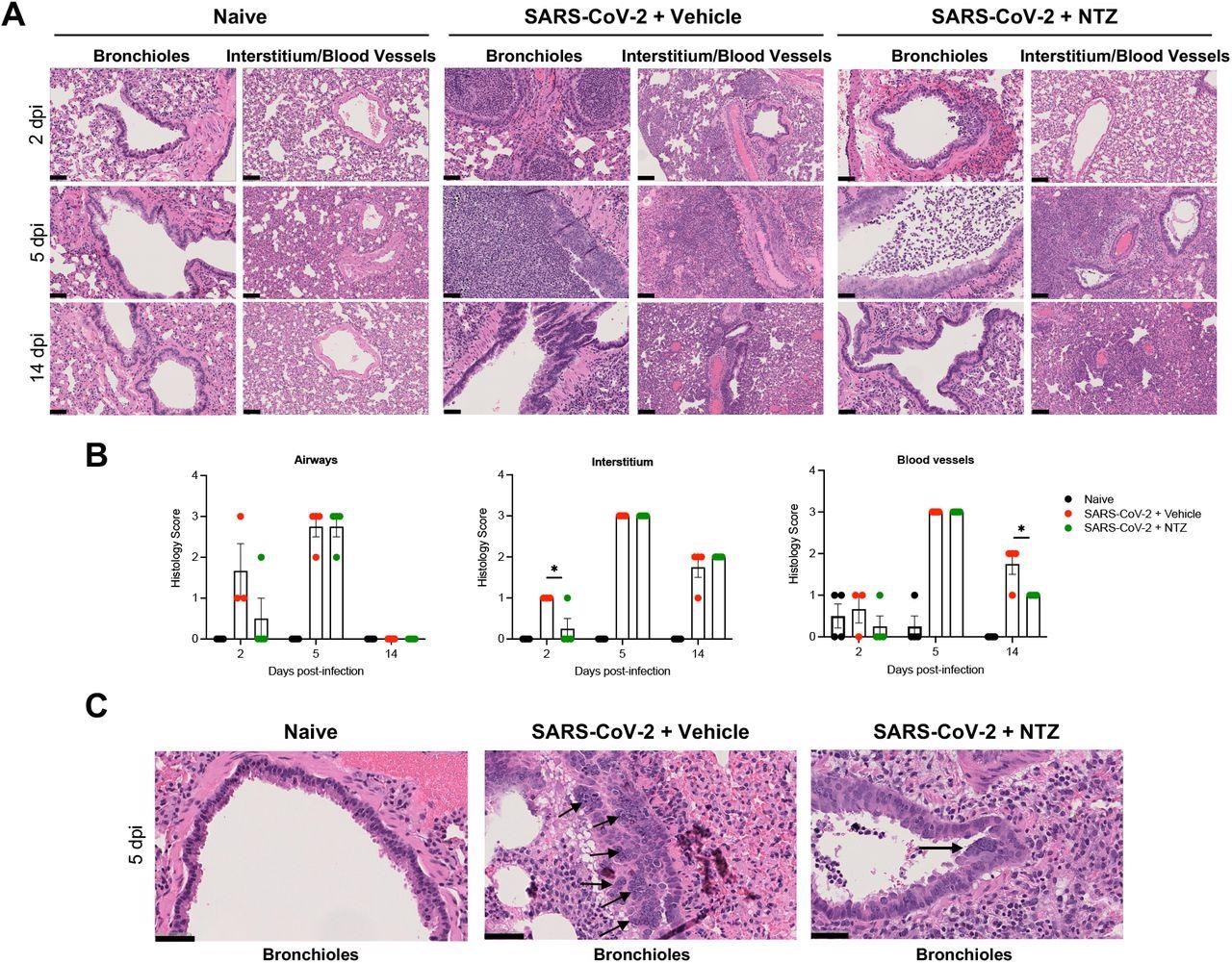
NTZ significantly prevents lung pathology SARS-CoV-2 infected Syrian hamsters. A. Comparison of normal lung (naïve) to pathologic changes induced by SARS-CoV-2 in vehicle-vs. NTZ-treated animals at day 2, 5, and 14 post-infection. Lung pathology at 5 dpi was severe irrespective of treatment group. B. NTZ significantly reduced interstitial inflammatory and perivascular scores at 2 and 14 days post-infection. Qualitatively there was decreased bronchiole epithelial injury and associated luminal inflammatory exudate. Lung pathology at 5 dpi was severe irrespective of treatment group. Asterisk indicates statistically significant differences by one-tailed unpaired Student’s t-test at day 2 for interstitium and on day 14 for blood vessels. Lungs from 4 animals were included in the scoring analysis for each timepoint except for 2 dpi, where there were 3 SARS-CoV-2 PBS/vehicle-treated animals. C. Bronchiole syncytial cells were observed exclusively at 5 dpi in SARS-CoV-2-inoculated animals and were more frequently observed in the vehicle-treated group. Scale bars: A, 100 μm; C, 50 μm.
Conclusions
In a human trial by Blum et al., 2021, after five days of treatment with NTZ, patients with moderate COVID-19 showed a substantial decrease in inflammatory biomarkers, faster recovery, and reduced hospitalization time. Another randomized controlled trial (RCT) in humans tested the efficacy of extended-release formulation of NTZ (NT-300) in mild or moderately ill COVID-19 patients. The results showed an 85% reduction in progression to severe illness after five days of treatment. Overall, these findings suggest that NTZ is safe for use in adults and children.
Therefore, an NTZ-based combination cocktail could lower the dosage requirement of expensive new antiviral oral drugs, especially for children and immunosuppressed HIV-infected individuals. Furthermore, its oral formulation is already widely available globally at an economical cost, making NTZ easily deployable and accessible.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- The oral drug nitazoxanide restricts SARS-CoV-2 infection and attenuates disease pathogenesis in Syrian hamsters, Lisa Miorin, Chad E Mire, Shahin Ranjbar, Adam J Hume, Jessie Huang, Nicholas A Crossland, Kris M White, Manon Laporte, Thomas Kehrer, Viraga Haridas, Elena Moreno, Aya Nambu, Sonia Jangra, Anastasija Cupic, Marion Dejosez, Kristine A Abo, Anna E Tseng, Rhiannon B Werder, Raveen Rathnasinghe, Tinaye Mutetwa, Irene Ramos, Julio Sainz de Aja, Carolina Garcia de Alba Rivas, Michael Schotsaert, Ronald B Corley, James V Falvo, Ana Fernandez-Sesma, Carla Kim, Jean-François Rossignol, Andrew A Wilson, Thomas Zwaka, Darrell N Kotton, Elke Mühlberger, Adolfo García-Sastre, Anne E Goldfeld, bioRxiv, 2022.02.08.479634; doi: https://doi.org/10.1101/2022.02.08.479634, https://www.biorxiv.org/content/10.1101/2022.02.08.479634v1
Posted in: Medical Research News | Disease/Infection News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Animal Model, Assay, Blood, Blood Vessels, Cell, Cell Line, Cell Sorting, Children, Compound, Coronavirus, Coronavirus Disease COVID-19, Cryptosporidium, Cytometer, Cytometry, Cytotoxicity, Diarrhea, Drugs, Efficacy, Enzyme, Flow Cytometry, Fluorescence, Giardia, HIV, Imaging, in vitro, in vivo, Interstitium, Lungs, Metabolite, Pandemic, Pathology, Preclinical, Receptor, Remdesivir, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Virus, Weight Loss

Written by
Neha Mathur
Neha Mathur has a Master’s degree in Biotechnology and extensive experience in digital marketing. She is passionate about reading and music. When she is not working, Neha likes to cook and travel.
Source: Read Full Article