Fox News Flash top headlines for December 10
Fox News Flash top headlines are here. Check out what’s clicking on Foxnews.com.
A Tennessee-based pharmaceutical company announced Wednesday that it is “voluntarily recalling” two drugs – one that treats erectile dysfunction, the other depression – after a “product mix-up” that resulted in the medications being "inadvertently packaged together" when they were bottled at a third-party facility.
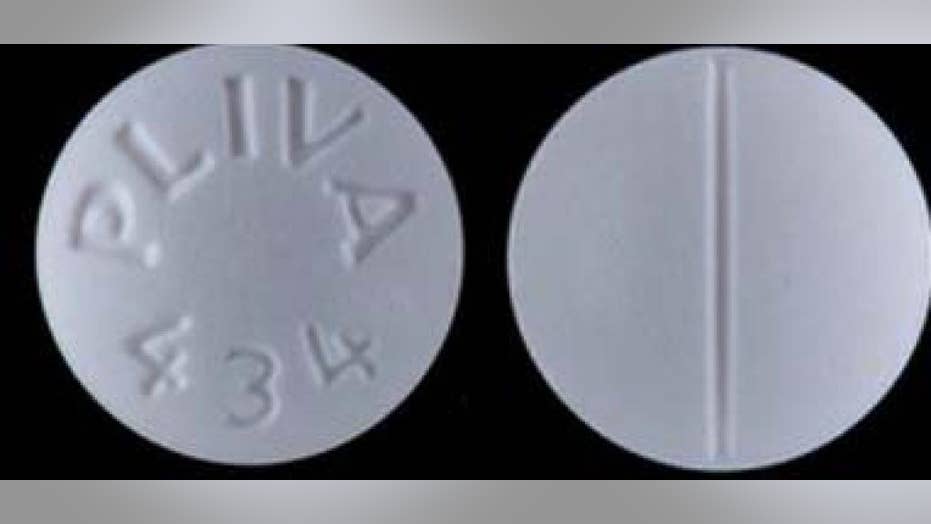
AvKARE, which is headquartered in Pulaski, Tenn., is recalling one lot of Sildenafil 100 mg tablets and one lot of Trazodone 100mg tablets to the consumer level, the company said in an announcement reposted to the U.S. Food and Drug Administration’s website.
Sildenafil is the active ingredient in Viagra, which is a PDE-5 inhibitor, and is used for the treatment of erectile dysfunction and is packaged in 100-count bottles, the company said.
ERECTILE DYSFUNCTION DRUGS MAY HELP REDUCE EARLY DEATH FROM COLON CANCER, STUDY FINDS

The recalled medications were "inadvertently packaged together."
(FDA)
Unintentional consumption of Sildenafil may pose serious health risks to consumers with underlying medical issues, according to AvKARE. For example, Sildenafil may interact with nitrates found in some prescription drugs, such as nitroglycerin, lowering blood pressure to dangerous levels. Consumers with diabetes, high blood pressure or heart disease often take nitrates.
The company said Trazodone hydrochloride is indicated for the treatment of major depressive disorder and packaged in 1,000- count bottles.

The impacted products were distributed nationwide.
(FDA)
Unintended intake of Trazodone may result in adverse health consequences, such as somnolence/sedation, dizziness, constipation and blurred vision – all adverse events that may be more concerning in elderly patients due to a subsequent increased risk for falls and driving impairment, according to AvKARE.
VIRAL TIKTOK CHALLENGE HIGHLIGHTS STRESS, DEPRESSION, ANXIETY TEST
The affected lots of Sildenafil 100 mg Tablet — Lot 36884 with an expiration date of 03/2022 — and Trazodone Hydrochloride 100 mg Tablet — Lot 36783 with an expiration date of 06/2022 – “were distributed to our distributors and wholesalers, and then further distributed nationwide,” the company said.
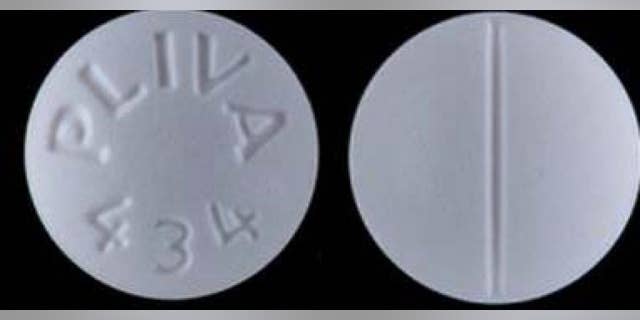
AvKARE said it has notified distributors and consumers of the error.
(FDA)
AvKARE added that it “notified its distributors and customers and is arranging for return of all recalled product of the listed lots.”
At the time the press release was published Wednesday, AvKARE had not received any reports of adverse events related to this recall.
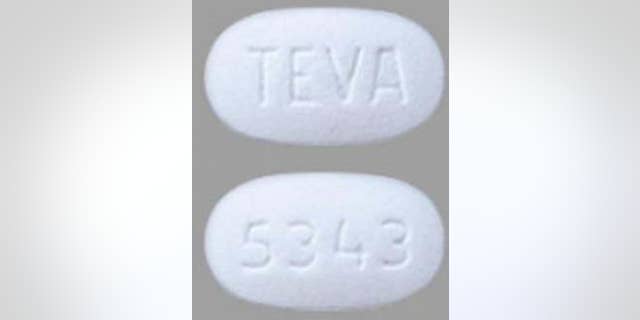
As of Wednesday, no adverse reactions related to the recall had been reported.
(FDA)
It advised any consumer who believes they have experienced any problems related to taking these drugs to contact their physician or health care provider. Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
CLICK HERE TO GET THE FOX NEWS APP
Source: Read Full Article



