A new study by University at Buffalo researchers has revealed that the absence of a single interaction within a brain receptor reduces its activity. The discovery advances the understanding of how certain brain diseases arise, and could lead to developing precision medicines for treating them.
The study was published Dec. 31 in Proceedings of the National Academy of Sciences by senior authors Gabriela K. Popescu, Ph.D., professor of biochemistry in the Jacobs School of Medicine and Biomedical Sciences at UB, and Wenjun Zheng, Ph.D., UB professor of physics in the College of Arts and Sciences; first authors are Gary Iacobucci, Ph.D., a postdoctoral fellow in Popescu’s lab, and Han Wen, a doctoral candidate in Zheng’s lab.
The research builds on more than a decade of work by Popescu, who studies the brain’s NMDA (N-methyl-D-aspartate) receptors. Mutations in this protein, which is critical to learning and memory, can result in neuropsychiatric diseases, from epilepsy to schizophrenia. Such mutations are rare and have only been discovered in the past 10 years.
Popescu’s work focuses on how subtle changes in these key receptors cause them to produce altered electrical signals, which in turn, affect how well the brain functions.
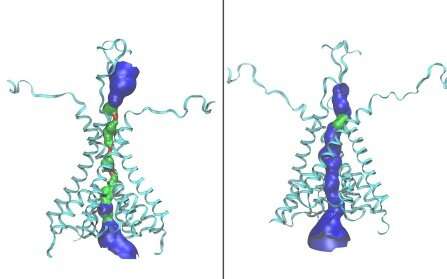
The work describes for the first time an open form of the NMDA receptor and identifies a direct interaction between two amino acid residues. This interaction forms only in the open receptor and helps it stay open for longer, a finding that has clinical implications.
“Cycling repeatedly through its open and closed forms is the main business of NMDA receptors,” explained Popescu, “and the amount of time that the receptors stay open or closed determines the strength and duration of the electrical signal they produce when stimulated.”
Excitability levels
The electricity generated by the opening and closing of the receptors, in turn, determines a neuron’s level of excitability, which has direct clinical consequences. “Too much excitability can mean epilepsy, seizures or neurodegeneration, whereas too little can result in schizophrenia and other cognitive disorders,” she said.
Until now, the structure of an open form of the NMDA receptor was unknown. To date, the literature has reported only atomic structures for juvenile NMDA receptors, present in young mammals or young neurons, and these are believed to represent a closed form of the receptor.
In previous work, Popescu collaborated with UB co-authors Wen and Zheng to develop a model of how the predominant NMDA receptor protein in the adult brain might look.
In the current paper, the two teams built upon that work and used molecular dynamics simulation to “force” the closed adult receptor to open. This was accomplished with the use of supercomputing power through UB’s Center for Computational Research and mathematical algorithms developed in the Zheng lab.
First clue
“This simulated open structure is the first clue to how the internal organization of these receptors may change when they open,” said Popescu.
When they compared the positions of atoms between the closed and open NMDA receptor structures, the researchers were able to identify several locations where two amino acid residues had moved closer together, suggesting that they were engaging in a new interaction.
“When people have receptors that cannot form this interaction, their receptors and synapses are more sluggish, not as active,” said Popescu. “The observations in this paper are consistent with symptoms observed in patients whose receptors lack this interaction due to spontaneous mutation of one of the residues we identified here as important.”
She noted that precision medicine for NMDA receptors is still in its infancy and the U.S. Food and Drug Administration has only approved a few drugs that work on these receptors.
“Functional studies like this will help us better understand how the various mutations affect receptor function and which therapy to try,” said Popescu.
Next steps
The researchers will continue to collaborate to better understand not just open and closed NMDA receptors, but also their intermediary conformations. Large genome sequencing studies will also be crucial in identifying the spectrum of mutations in people and revealing how specific mutations lead to characteristic symptoms.
Source: Read Full Article



